Field vs Pot Sporulation
Pot Cultures
Why does sporulation occur abundantly in pot cultures and more rarely in the field?
Over the years, and more than 1000 trap cultures from field soils, we have seen a trend where fungal species not detected in the field because sporulation is absent or too low to measure, produce moderate to abundant spores in mature pot cultures (growing 4 months or longer with a C4 grass host). This pattern is especially common to samples obtained from (i) pastures, (ii) semi-arid sites, and (iii) many native grassland sites. This trend is not universal, however, and that contributes to our understanding of causation.
In some field communities, sporulation can be as high or higher than that obtained in greenhouse pot cultures. In general, these sites include (i) sand dunes, (ii) wetlands, and (iii) acidic minesoils, where plant distribution is patchy with open areas devoid of any growth. These patterns indicate that interplant dynamics (with heavy involvement of interplay amongst root systems) and soil environments are important players in the relationship between fungal growth (mycorrhizal development) and sporulation. Outcomes from different pot culture protocols used by the collection provide additional clues:
- Addition of autoclaved oat kernels to a potting medium stimulated shoot (and root) growth of seeded plants and a 30-40 day delay in sporulation compared to the same system without oat kernels. Mycorrhizal colonization (measured as mycorrhizal root length) in the former was almost 1.5 times that in the latter. Abundance of sporulation, in spite of temporal differences, was similar in both treatments.
- Corn growing 8 liter pots produced more plant biomass (taller, wider leaves, greater stem diameter) than corn grown in 1.5 liter pots. This result is a natural expectation. However, onset of fungal sporulation was delayed 35-45 days in the larger pot.
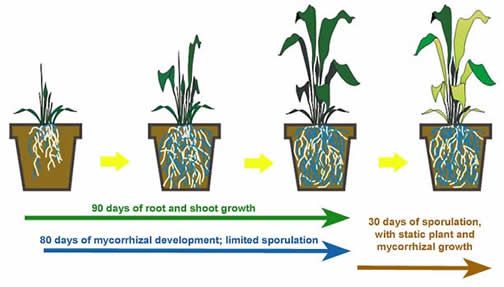
- Sudangrass grown in 15-cm diameter pots (approx. 1.5 liters) and colonized by either Gigaspora margarita or Dentiscutata heterogama produced abundant hyphae, auxiliary cells, and a low number of spores at 90 days. After an additional month in which plant growth had become static, auxiliary cells were rare and spore abundance increased 10-fold or more.
Hypothesis
From the observations summarized above, we hypothesize that the following is occurring in pots:
- During the active phase of shoot and root growth, most of the carbon is going to the fungi as they colonize roots and investing in mycorrhizal development. In other words, fungal growth takes advantage of an ever expanding root niche space. Carbon also is allocated to external hyphal development, but mostly for hyphae that are infective (producing secondary units of colonization for further spread). The fungi are not partitioning much carbon to sporulation, possibly because sink size would compromise rate and amount of mycorrhiza biomass being produced.
- Once roots have ceased growth because of constraints imposed by pot boundaries, mycorrhizal colonization catches up with root niche space and at some point also reaches stasis.
- With continued photosynthesis in shoots, carbon is repartioned to the one niche still open to fungal growth, and that is in sporulation. After all, spores are not reproductive units but instead are another component of the fungal thallus capable of clonal growth. This allocation continues as long as there is an active carbon source (leaves, stems). The extent of continued sporulation following stasis in plants seems to be consistently greater and longer for Gigaspora, Scutellospora, Racocetra, Cetraspora, and Dentiscutata species than for genera forming glomoid spores (e.g. Glomus, Claroideoglomus, Funneliformis, Rhizophagus, Diversispora, Paraglomus), acaulosporoid ( Acaulospora, Archaeospora) or both ( Ambispora).
Reference
- Pringle A. & J. D. Bever. 2002. Divergent phenologies may facilitate the coexistence of arbuscular mycorrhizal fungi in a North Carolina grassland. American Journal of Botanyz 89: 1439-1446.