Infectivity Assays
Several bioassays have been developed to indirectly measure the number (or relative amount) of infectious fungal propagules in a sample using plant seedlings as host and measurement of mycorrhizal colonization after a defined period of time as the data. While three methods are described here, the MPN and MIP assays are generally the only ones widely used.
described here, the MPN and MIP assays are generally the only ones widely used.
Factors Affecting Infectivity Assays
Infectivity assays have four component factors which must each receive scrupulous consideration: (1) plant host, (2) plant growth medium and the size of pots in which the medium is placed, (3) fungal inoculum, and (4) environmental conditions. Each will be discussed separately here.
Plant Host
Numerous plant species can be used in infectivity assays, since most have some dependence on the mycorrhizal association and will become colonized. The most dependent plant species are preferred to optimize opportunity for mycorrhiza establishment with contact between fungal propagules and roots. Balanced against this consideration is that of root geometry. Most assays are of short duration, so fungus-plant contact is improved with plants having a fine fibrous root system that rapidly infiltrates all of the volume of the growth medium. C4 grasses (e.g. bahia grass for tropical climates, sorghum, sudangrass, or big bluestem for temperate environments) have these properties. Legumes also are good hosts, but many genotypes are highly susceptible to ubiquitous insect pests such as mites and white flies and they also tend to increase nematode numbers and activity.
Plant Growth Medium/Container Properties
The main physical property to worry about is size distribution of particles to keep bulk density low but maintain good aeration. Without these conditions, root growth will not occur evenly throughout a pot or tube and frequency of root-propagule contact will be severely reduced. Sand works well when mixed with soil (we mix it 2:1 v/v with a loamy soil prior to steaming).
Chemical properties include adequate nutrients (with phosphorus level kept low to stimulate mycorrhizal development) to balance vigorous root growth with optimal host compatability to mycorrhiza formation. Concentrations of cations should be adjusted to provide the appropriate pH, exchange capacity, and other conditions that may influence infectivity, which in turn would be determined by soil conditions at the site where the fungus of interest was discovered.
Choice of the type of growth medium depends on the goals of the infectivity assay:
- If the aim is to determine infectivity of propagules in an environment similar to that from which the fungus was collected, then the source field soil should be used (mixed with sand to improve aeration) after sterilization at a minimum temperature of 80oC. To minimize toxicity problems associated with increases in manganese concentrations accompanying heat sterilization, the growth medium should be dried and rewetted several times before use. It is a good practice to “naturalize” the soil environment by reintroducing native microbial populations (the liquid that passes through a fine sieve with 8-10 micrometer openings).
- If the aim is to determine infectivity of fungal propagules in a particular experimental soil, then the assay growth medium should be of identical composition. The same considerations discussed above for field soils apply to these as well.
The containers for growing the plants should have a high length/width ratio and hold a small volume to maximize propagule-root contact in assays of short duration. The long slender plastic growth tubes (2.5×12.5 cm, a 50 cm3 volume) sold as “Ray Leach Cone-Tainers” by Stuewe and Sons, Inc. (2290 S. E. Kiger Island Drive, Corvallis, Oregon 97333) are ideal because of their small volume. Alternatively, PVC pipe with nylon or other inert substance closing the lower end can be used.
Fungal Inoculum
Inoculum of arbuscular and vesicular-arbuscular mycorrhizal fungi consists of different types of infective propagules: spores, vesicles (members of Glomineae only), hyphal fragments, and hyphae from mycorrhizal root pieces. While some research has indicated that hyphae and roots are most infective, this conclusion cannot be generalized to all isolates of a species or even to all species (see L. Abbott and A. Robson, 1981 Aust. J. Agric. Res. 32:631-639).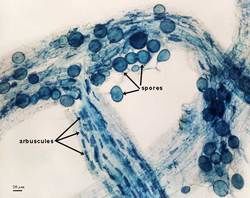
Spores can be separated from other propagules by wet-sieving and sucrose density gradient centrifugation to assay separately. This goal becomes important when (i) spores are used alone as inoculum in culture development or in experiments or (ii) spores are extracted and the population is to be added to an inert medium for inoculum storage and subsequent use. However, spore infectivity assays are complicated by the influence of spore recovery operations (extraction, manipulation, storage) as well as endogenous and exogenous constraints on germination potential. To minimize these factors, it is probably a good idea to incubate harvested whole inoculum in the refrigerator or at room temperature (temperate and tropical isolates, respectively) for at least one month prior to spore extraction. Spores extracted in sucrose should be stored in distilled water at least 24 hours before mixing them with the growth medium to allow them to overcome osmotic shock. Spores must be mixed thoroughly with the growth medium to prevent interference or competition effects. Dilutions can be carried out two ways: (i) spores diluted serially in distilled water, each dilution then mixed with the growth medium or (ii) spores added to the growth medium, which is then serially diluted with sterile medium. An ideal container for mixing spores in a solid substrate are gallon-size “Zip-loc” bags filled with air before they are sealed.
Whole inoculum collected from a field site should consist of different soil cores that then are pooled. This practice makes certain that heterogeneity of the site is suitably represented. Whole inoculum from pot cultures should consist of aliquots sub-sampled after roots and growth medium have been chopped up and thoroughly mixed. Roots must be broken into small fragments to insure proportional reductions during dilution.
Environmental Conditions
Environmental conditions impact on the physiology of the plant host, soil chemical properties, and physiological state of the fungal propagules. These complex interactions all influence infectivity and resultant mycorrhizal development. Only under completely controlled conditions (e.g. quality plant growth rooms) can assays carried out at different times and compared. In greenhouses, where seasonal variables cannot be fully controlled, assays must be set up so that all relevant comparisons are made in the same experiment. Even then, conditions should be kept as uniform as possible (especially temperature, moisture, and light).
Standardization of experimental conditions does not translate to standardization of environmental conditions to which different fungi will react. Please keep in mind that each fungus in an inoculum or each inoculum (if from different soil environments) may “see” the same host and the same medium in which it is placed differently and react accordingly. For this reason, it is important to keep in mind whatever disparities exist between assay conditions and those at the site(s) where the test fungi resided.