Amorphous
Spore Morphological Characters
Spores of glomeromycotan fungi have been described using a bewildering array of “walls”, “groups”, “components” that are unique among members of the Kingdom Fungi. These structures were known to be taxonomically important because they are highly conserved and phenotypically stable in almost any environment. Walker (1983) was the first to try and make sense of all the confusion, as new species were being described at that time based on any detectable difference regardless how of how small. He organized the various discrete phenotypes of spore subcellular structures into “wall” classes and then depicted each “wall” in murograph form for standardization and ease of comparison. The groundwork he laid was widely accepted at the time and the terminology was expanded as new “wall” phenotypes were discovered. Later, he and co-workers defined this structures as “components” of a spore.
Phenotypically Distinct Structures Used as Taxonomic Characters
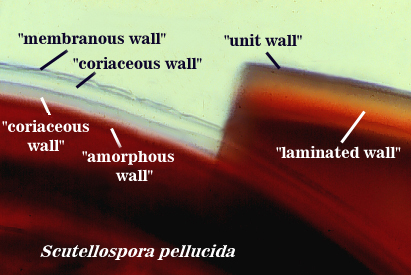
Unit Wall
| Gigaspora albida | Glomus clarum |
|---|---|
 | 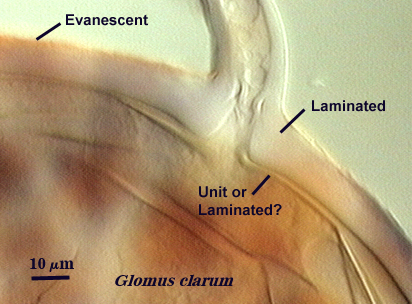 |
Walker (1983) defines this structure as “a single-layered, rigid wall clearly distinguished from others and consistent among spores of the same state of maturity within a species”. It can be found in species of all genera, although it often is confused with a laminated wall when the laminations are so adherent that they cannot be discerned. Walker even interpreted in his classic paper the laminated wall of A. spinosa incorrectly as a unit wall. Thus, the distinction can be a difficult one in some species. Moreover, immature spores in Glomus species only have what appear to be unit walls in youth, but these walls then become “laminated” (as new layers are laid down) or “evanescent” as they degrade and slough. As an example, compare the two spores of different isolates of G. clarum at right, where the inner wall varies in the extent to which layers are synthesized.
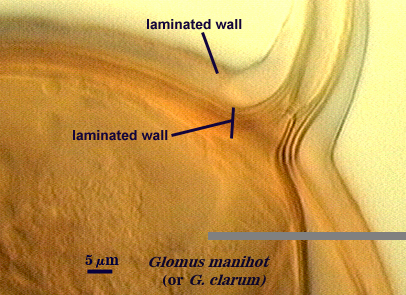
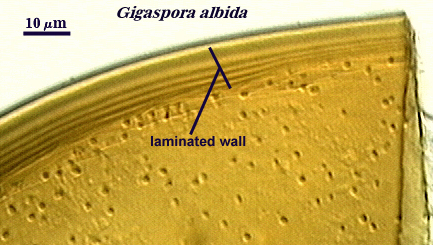
Laminated Wall
Walker (1983) defines this structure as “A rigid wall made of several layers laid down as the spore matures. Such a wall will have an increasing number of layers as the spore ages.” Recognition of this wall was fairly clear-cut as long as the laminations were laid down in such a way that they could be resolved at the light microscope level. However, this criterion varied greatly among species and genera.
Evanescent Wall
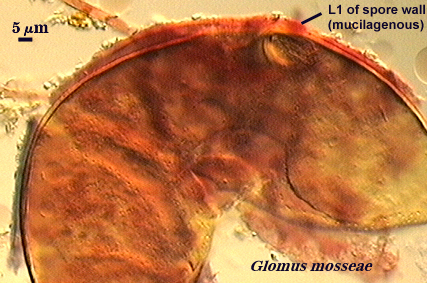
Walker (1983) defines this structure as “a unit or laminated wall that breaks down and sloughs as the spore matures”. Phenotypes that could be classified as laminated or membranous walls also may be evanescent. Within this definition are embedded many different phenotypes resulting from compositional differences among mostly “unit” walls, like the one on the spore of G. mosseae at right which has mucilagenous properties and stains dark red to brownish-red in Melzer’s reagent.
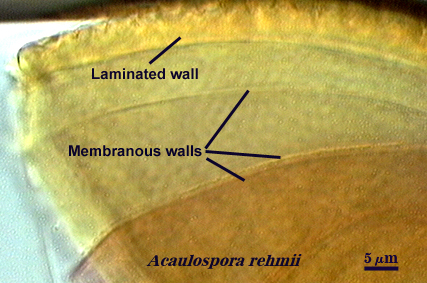
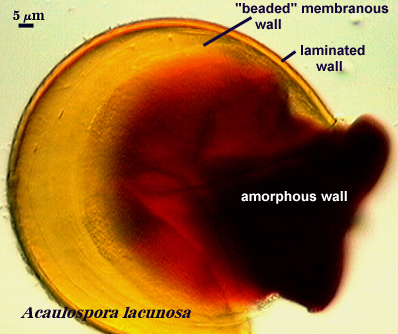
Membranous Wall
Walker (1983) defines this structure as “a very thin, often colorless, wall that often wrinkles and collapses in hypertonic solutions. Being flexible, it often does not break when a spore is crushed.” The surface of these walls may be smooth or “beaded”. This definition embodied structures that looked very similar (thin, colorless, folding), but which had very different origins and relationships with neighboring structures.
Coriaceous Wall
Walker (1986) defines this structure as a colorless wall in spores that is thicker than a membranous wall, but also is flexible and thus tough to break. It was described a having a leather-like appearance in hypertonic solutions. The problem with this structure is that the distinction between it and a membranous wall sometimes was difficult because of overlapping variation and phenotype.
Amorphous Wall
Morton (1986) describes this wall as being a colorless wall inside spores that is highly plastic with applied pressure to crushed spores in acidic mountants like PVLG or lactophenol. It stains red-purple to dark red-purple in Melzer’s reagent. The definition of this structure is based on behavior in an acidic environment, and thus distorts its true representation in nature. In reality, it is just a thicker wall with longer-chained glucose moieties than a coriaceous wall (which in turn is just a thicker membranous wall).
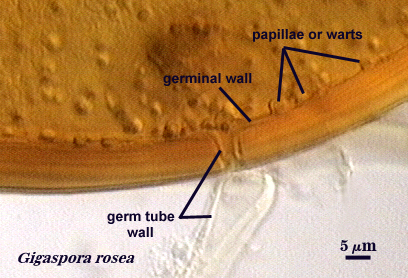
Germinal Wall
Spain et al. (1989) describe this wall as the innermost wall only in spores of Gigaspora species. It is of similar phenotype to layers of the laminated wall, but forms warty protruberances or papillae prior to germ tube formation. This is the only wall named for its function rather than structure (which is obvious only at the ultrastructural level). There really is no evidence that this is a separate wall; it appears to have been defined to highlight its difference in function from the laminated wall.
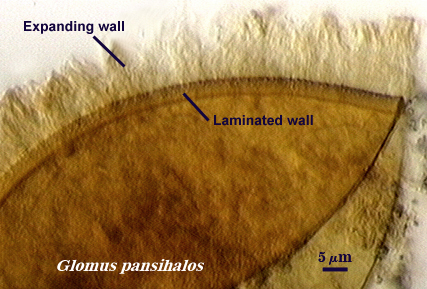
Expanding Wall
Berch and Koske (1986) describe this wall as a unit wall which expands and produces perpendicular striations in some mountants, such as lactic acid or polyvinyl alcohol (PVLG). It must be the outermost wall of a spore for the phenotype to be expressed. This wall probably is the most dubious because it is likely an artefact of spore state (most were parasitized) and mounting conditions. In reality, it represents a unit wall that has a chemical structure which responds to acidic conditions by expanding and altering its appearance in the process. It has been found only in Glomus pansihalos and possibly Glomus coronatum.
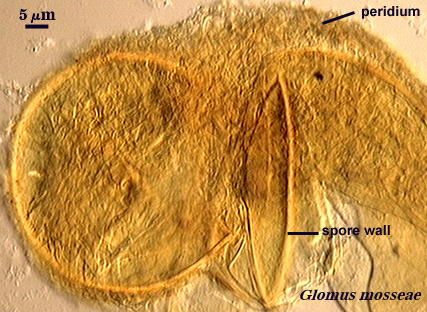
Peridium
In some species of Glomus and Sclerocystis (for those who fail to see the continuum in organization of spores of similar formative stages), spores develop a hyphal network around individual spores and in small to large aggregates of spores. This network presumably arises from the subtending hypha, although critical developmental studies are needed.
Structural Complexes Used as Taxonomic Characters
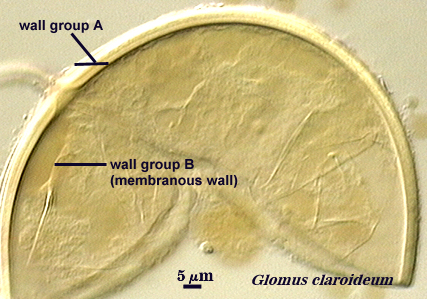
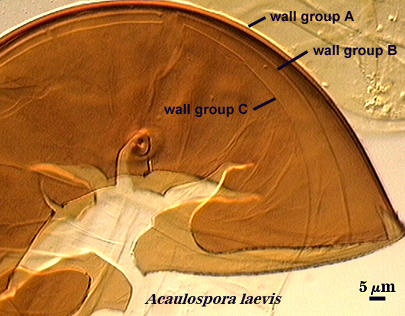
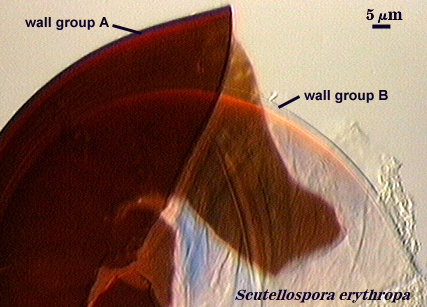
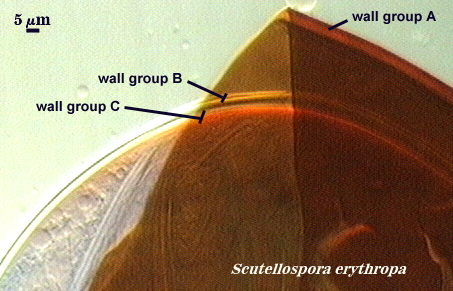
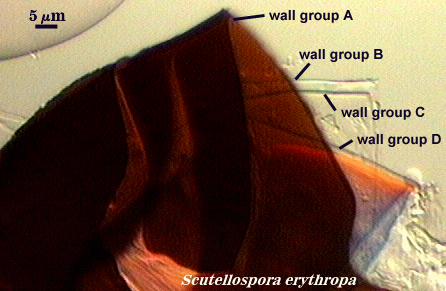
Walker (1983) developed the concept of “wall groups”. He defined a wall group was defined as “an aggregation of walls that are either adherent, or that remain close together when a spore is crushed”. This concept proved to be subject to much variation and interpretation because the degree of separation often was influenced greatly by condition of spores (fresh, fixed, parasitized, aged), amount of pressure applied to a spore when it was crushed on a slide (light, heavy, super-squashes), and the type of mountant in which spores were placed. The interpretational difficulties are illustrated in the photos below. In G. claroideum spores, for example, the “membranous inner wall” actually is a part of the hyphal wall and remains attached to it and the “laminated wall” of which it also is a part. The four “wall groups” of S. erythropa can appear as two (left photo in a series of three), as three (two of the four are too close together, as depicted in the middle photo) or as four definite groups (right photo). If the spores are preserved, then only the two wall groups are usually seen. In healthy specimens, one finds the full range of variation. In Scutellospora species with fewer walls (e.g., S. fulgida below) or in many Acaulospora and Entrophospora species, wall groups tend to be more consistent among spores of the same population.
| Wall Groups | ||
|---|---|---|
 |  | 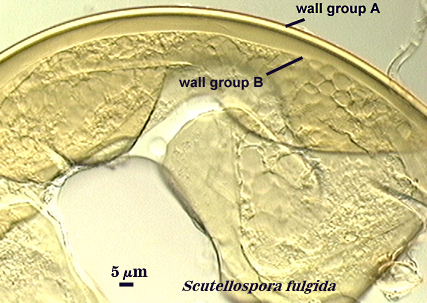 |
| Two, three, and four wall groups (left to right) in Scutellospora | ||
|---|---|---|
 |  |  |
While the above-mentioned wall definitions served a very important function during the 1980’s to serve as an organizing principle for delimitation of taxonomic characters, they possessed two fatal flaws in the long-term.
- Definitions were too “typological”, so that they had to either change to accommodate new variants or new definitions had to be erected.
- There was no connection between structure and any biological process, such as development. Without such a connection, the definitions were artificial and hence unusable for systematic (phylogenetic) approaches to understanding a natural taxonomic hierarchy. The problem became acute when cladistic analysis (Morton, 1990) indicated that some of the defined structures were not truly independent of each other, but rather were components of more complex structures. Consider, for example, that all of the wall definitions do not distinguish between a spore wall and any other wall that may have a separate origin (forming an endospore) or function.
The problems encountered with this terminology were correctable with developmental studies to define spore structures by their temporal and spatial origin, their transformational states between origin and maturity, and their relationship to neighboring structures. Armed with this data, other comparative studies then were initiated to determine if the patterns observed extended to species in other genera and families. These studies led to a developmental model for Glomales and new terminology which reflected the nature of each structural character and it relationship to those surrounding it.
References
- Berch, S. M. and Koske, R. E. 1986. Glomus pansihalos, a new species in the Endogonaceae, Zygomycetes. Mycologia 78: 832-836.
- Morton. J. B. 1986. Three new species of Acaulospora (Endogonaceae) from high aluminum low pH soils in West Virginia. Mycologia 78: 641-648.
- Spain, J. L., Sieverding, E. and Schenck, N. C. 1989. Gigaspora ramisporophora: A new species with novel sporophores from Brazil. Mycotaxon 34: 667-677.
- Walker, C. 1983. Taxonomic concepts in the Endogonaceae: spore wall concepts in species descriptions. Mycotaxon 18: 443-455.
- Walker, C. 1986. Taxonomic concepts in the Endogonaceae: II. A fifth morphological wall type in endogonaceous spores. Mycotaxon 25: 95-99.