New Terminology
Developmental Concepts of Morphological Characters
The developmental concepts presented on this page were generated from exhaustive comparative studies of fungal isolates of selected species in all genera grown in pot cultures. Only recently have these data provided a pattern which is predictable for all species encountered in the collection. This pattern is being used to construct a model universal for all Glomeromycota which defines the level of resolution of each discrete morphological character. The model is complex, and so it will not be explained in its entirety until all the details have been published in the primary literature. Publications of this work done at INVAM are:
| Term | Description |
|---|---|
| Gigasporaceae | Benivenga and Morton (1995) Franke and Morton (1994) Morton (1995) |
| Glomeraceae | Morton (1996) Stürmer and Morton (1997) |
| Acaulosporaceae | Stürmer and Morton (1998) |
| Archaeosporaceae | Morton and Redecker (2000) |
The universal pattern for differentiation of subcellular structures in glomalean spores is distinctly hierarchical, in that there are structures that are made up of component parts and each component part has unique phenotypic properties. It is this hierarchy which starkly contrasts with previous taxonomic concepts. Once it is understood, however, interpretations of taxonomic structures are more intuitive and predictable. The focus on this page is morphological properties of spores to “keep it simple” and because a majority of the evolutionary conserved features are present therein.
1. Spore Wall
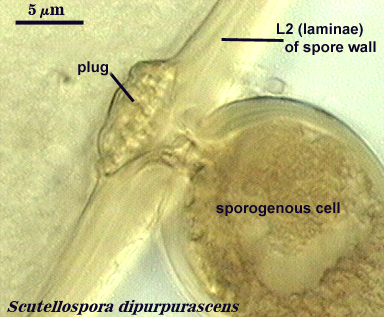
Each spore, regardless of species, produces one spore wall which originates from the fertile hypha on which the spore is borne and either remains continuous with the hyphal wall or becomes detached (sessile). This spore wall grows and differentiation's mostly while the spore is expanding in size. When the spore ceases to expand, then all layers of the spore wall have formed, and they may continue to differentiate properties such as color, rigidity, and some thickening to a limited extent. The spore wall as a single entity does not exist in taxonomic concepts now generally accepted. Below are photographs depicting the continuity between layers of the hyphal wall and that of the spore wall. In Glomus, the link between the hypha and spore wall is most obvious during spore development because both hyphal and spore walls differentiate together and at the same rate (see Morton, 1996; Stürmer and Morton, 1997).
| Various Spore Walls | ||||
|---|---|---|---|---|
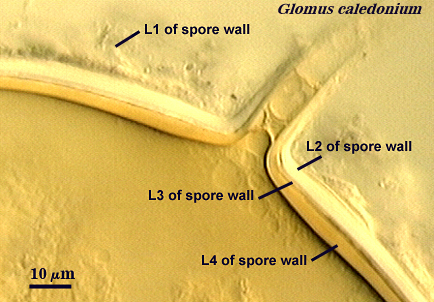 | 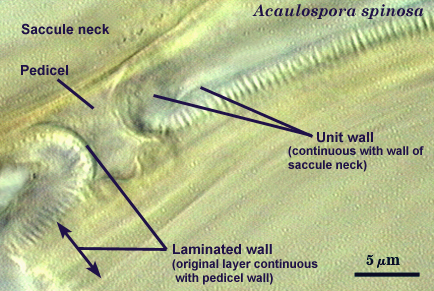 | 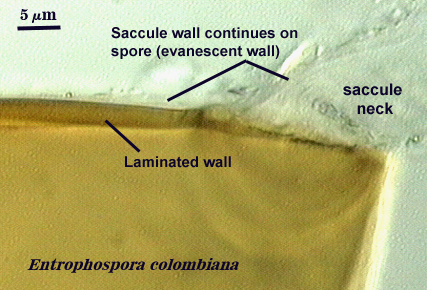 | 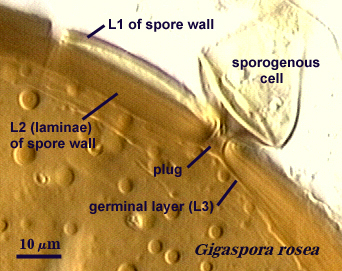 |  |
A. Layers of the Spore Wall
Within each spore wall, two or more (although rarely more than four) layers are synthesized (see labels in photos above). Position is the most important information amongst layers (or where is one located relative to all others present). In the pages on this website, therefore, these layers are numbered from outermost to innermost (L1, L2, L3, etc.). Layers are not defined by the order in which they are formed (although most develop from outer to inner surface of the spore) because developmental studies should not need to be conducted by anyone trying to make an identification or to describe a new species. Some of the more commonly distributed layers are synonymous with various “wall” types of present-day taxonomic concepts; others are new and have been included in descriptions but not formally defined as discrete “walls”.
Question: What distinguishes a wall from a layer?
Answer: A wall has a unique origin in the development of the spore, whereas layers represent component parts that share a common origin. The distinction is determined empirically by temporal and spatial appearance of each structure relative to the one before it and the one after it in developmental comparisons by the taxonomist defining the species.
B. Properties of Each Layer in the Spore Wall
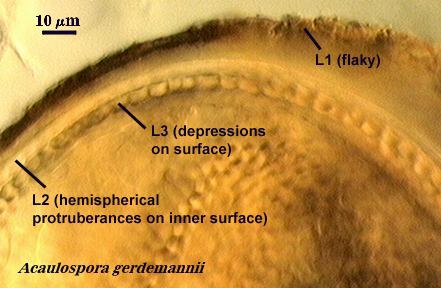
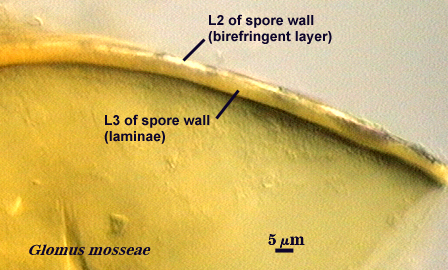
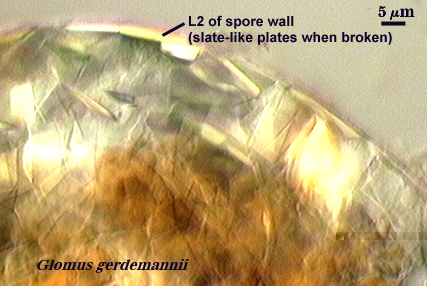
It is here that developmental concepts also deviate from the established paradigm. Phenotypes represent divergent properties of layers and represent most of the evolutionary innovation that has occurred. The range of phenotypes is much less predictable than the number of layers, and so we are likely to find new phenotypes as additional species are found. There is prediction, however, among some layers with certain phenotypes with regard to their position in the spore wall and the extent to which they are permanent or degrade. Structures previously termed “unit walls” are actually layers of the spore wall with many different phenotypes, such as mucilagenous, slate-like, granular, swollen in acid (= “expanding wall”) and so on. They also may be permanent or sloughing (= “evanescent wall”). A layer which forms few to numerous sublayers (common origin and of similar properties) as it grows and differentiates is synonymous with the “laminated wall”. We often refer to this layer as a “laminate layer” with a variable number of “sublayers” for continuity in terminology. In some species a thin layer on the inner surface of the spore wall separates, which because it is both thin and flexible (both traits are linked, understandably), resembles a flexible inner wall (called previously a “membranous wall”). Such a layer is present in species of most genera, although it is not always obvious. It is recognized as a part of the spore wall because it originates as part of the wall of the subtending hypha in all genera except Acaulospora and Entrophospora.
| Various properties of spore wall layers recognized conventionally as "unit or evanescent walls". | ||
|---|---|---|
 |  |  |
2. Flexible Germinal Wall
A flexible germinal wall (previously called “inner” wall) is so named because it folds to varying degrees depending on its thickness and the extent to which pressure is applied to the spore when it is crushed under a coverslip and it is linked contemporaneously or historically with formation of a “germination orb” (Acaulosporaceae) or a “germination shield” (Gigaspsoraceae). Unlike the spore wall, it is not naturally pigmented and thus sometimes may be hard to see if it is adherent to the spore wall (a common occurrence in fixed, preserved, or aged specimens). They are distinct from the spore wall in that they arise de novo AFTER synthesis of all layers of the spore wall have completed synthesis, and they have no physical connection to the parent subtending hypha. This structure is synthesized exclusively in species of Acaulospora and Entrophospora (Acaulosporaceae) and Scutellospora (one of two genera in Gigasporaceae). Spores of Gigaspora (Gigasporaceae), Glomus (Glomaceae), Paraglomus (Paraglomaceae) and Archaeospora (Archaeosporaceae) species examined to date show no evidence of producing any flexible germinal walls.
Layers of a Flexible Germinal Wall
Most flexible inner walls consist of two layers (numbered consecutively from outer to inner as L1 and L2) that previously have been treated as independent structures defined as a “membranous wall”, “coriaceous wall”, or “amorphous wall” (see photos above). When more than one wall is formed, each arises independent of the other, with the second forming only after the first has completed differentiation of all its layers. Since both layers of a wall are of common origin, they often are more adherent to each other than to a separate flexible inner wall. That appears to be the development basis for the occurrence of “wall groups”.
Properties of Each Layer in a Flexible Germinal Wall
Visible properties in each layer of a flexible inner wall appears involve mostly three interrelated properties: a) degree of thickness, b) degree of flexibility, and c) reaction in Melzer’s reagent. Some layers do not react in Melzer’s reagent regardless of thickness, probably because of differences in composition. For those that do react, generally the thinner the wall, the greater the capacity to fold and the weaker the reaction in Melzer’s reagent. The greater the degree of plasticity (“spreading” of the layer after several crushes), the greater the thickness (obviously) and the darker the staining reaction in Melzer’s reagent. The other significant property is occurrence of “beads” or “granular excrescences” on the surface of the outer layer of the innermost flexible wall in spores of Acaulospora and Entrophospora species (regardless of how many other flexible inner walls may be present). The “beads” tend to break away from the wall surface and disperse or they lose refractivity and appear to disappear after only a few days in a mountant (Morton, 1986). If there is some question about the presence of these excresences, spores can be stored in formalin for 60 days after which they become fixed to the flexible wall and stay attached regardless of how much pressure is applied to a spore.
3. Pregermination Structure
Formed as a requisite precursor to germ tube formation. It differs in design and position among genera of families in Glomeromycota.
References
- Bentivenga, S. P. and J. B. Morton. 1995. A monograph of the genus Gigaspora incorporating developmental patterns of morphological characters. Mycologia 87: 720-732.
- Franke, M. and J. Morton. 1994. Ontogenetic comparisons of arbuscular mycorrhizal fungi Scutellospora heterogama and Scutellospora pellucida: Revision of taxonomic character concepts, species descriptions, and phylogenetic hypotheses. Canadian Journal of Botany 72:122-134.
- Morton, J. B. 1986. Effects of mountants and fixatives on morphology and Melzer’s reaction of spores of two Acaulospora species (Endogonaceae). Mycologia 78:787-794.
- Morton, J. B. 1995. Taxonomic and phylogenetic divergence among five Scutellospora species (Glomales, Zygomycetes) based on comparative developmental sequences. Mycologia 87:127-137.
- Morton, J. B. 1996. Redescription of Glomus caledonium based on correspondence of spore morphological characters in type specimens and a living reference culture. Mycorrhiza 6:161-166.
- Stürmer, S. L. and J. B. Morton. 1997. Developmental patterns defining morphological characters in spores of species in Glomus (Glomales, Zygomycetes). Mycologia 89:72-81.
- Stürmer, S. L. and J. B. Morton. 1999. Taxonomic reinterpretation of morphological characters in Acaulosporaceae based on developmental patterns. Mycologia 849-857.