Paraglomaceae - J.B. Morton & Redecker
Biochemical Characters
Studies of biochemical characters, such as monoclonal antibody specificities (Wright et al., 1987) and fatty acid profiles (Graham et al., 1995) have led to the long-held suspicion that members of this family were phylogenetically distant from species in Acaulosporaceae and Glomeraceae, but all of these data did not contain enough phylogenetic information to determine it’s position relative to other glomeromycotan taxa. Small subunit ribosomal DNA sequences (SSU) provided the character set to position this family is ancestral to both families (Redecker et al., 2000; Sawaki et al., 1997).
Vegetative structures consist of those inclusive in the . However, some states of mycorrhizal structures are unique to this family.
Molecular characters which appear to be diagnostic for members of this family:
- Monoclonal antibody cell lines B5 and F8, maintained in the laboratory of Sara Wright (Wright et al., 1987).
- Signature DNA sequence at the 3’ end of the 5.8S ribosomal gene: GGCATGTCTGTTTGAGGGCACCA separates members of this family from those of Glomeraceae.
- The primer sequence TGCTAAATAGCCAGGCTGY (ARCH1311) also will selectively amplify species of this family.
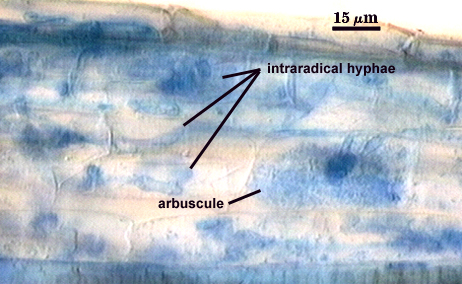
Arbuscules
Arbuscules stain very faintly or are almost invisible in conventional acidic stains. In at least one species (P. brasilianum), staining properties are more variable, with some regions of moderate staining intensity. Trunks < 4 µm wide, with fine branching near tips. Contrast in photo at right digitally enhanced to better see structure.
Vesicles

Vesicles are poorly characterized. None are observed in any isolates of P. occultum, but lipid containing structures (possibly spores) have been detected with sporadic distribution in roots containing P. brasilianum.
Extraradical Hyphae | |
|---|---|
 |  |
Extraradical hyphae staining darkly, 2-6 µm wide, often in profuse abundance around roots with attached spores.
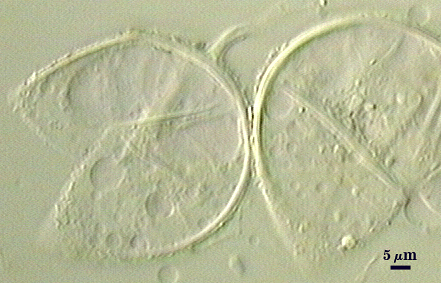
Asexual Spores
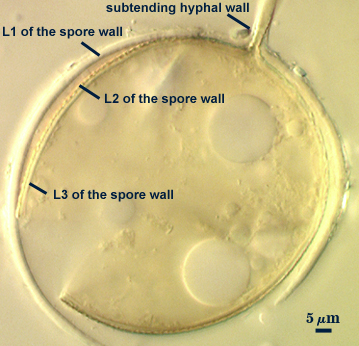
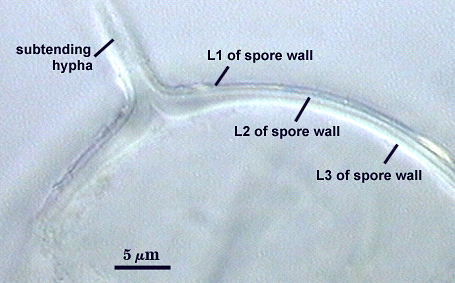
Species produce asexual spores which produced singly, more rarely in loose aggregates of 2-3 spores; subcellular structure is identical to that of Glomus species, with layers of the spore wall continuous with layers of the subtending hypha.
Develop terminally on a cylindrical to slightly flared subtending hypha.
Thickening Spore Wall
Spore is occluded in species described to date by thickening of the innermost spore wall layer.
Germination is poorly characterized in this group, but assumed to be like that in species of Glomeraceae: germ tube emergence through the lumen of the subtending hypha or more rarely through the spore wall.
References
- Graham, J.H., N. C. Hodge, and J. B. Morton. 1995. Fatty acid methyl ester profiles for characterization of glomalean fungi and their endomycorrhizae. Applied and Environmental Microbiology 61:58-64.
- Morton, J. B. and G. L. Benny. 1990. Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): A new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon37:471-491.
- Morton, J. B. and D. Redecker. 2001. Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93:181-195.
- Redecker, D., J. B. Morton, and T. D. Bruns. 2000. Ancestral lineages of arbuscular mycorrhizal fungi (Glomales). Molecular Phylogenetics and Evolution 14:276-284.
- Sawaki, H, K. Sugawara, and M. Saito. 1998. Phylogenetic position of an arbuscular mycorrhizal fungus, Acaulospora gerdemannii , and its synanamorph Glomus leptotichum, based upon 18S rRNA gene sequence. Mycoscience 39:477-480.
- Wright, S. F., J. B. Morton, and J. E. Sworobuk. 1987. Identification of a vesicular-arbuscular mycorrhizal fungus by using monoclonal antibodies in an enzyme-linked immunosorbent assay. Applied and Environmental Microbiology 53:2222-2225.