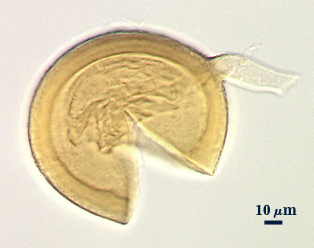
Rhizophagus fasciculatus
Whole Spores
COLOR: Pale yellow to pale yellow-brown
SHAPE: Globose, subglobose.
SIZE DISTRIBUTION: 60-110 µm

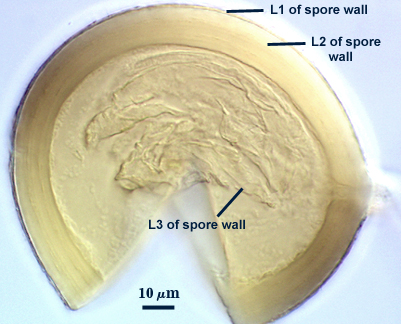
Subcellular Structure of Spores
Consisting of three layers (L1, L2, and L3) which form sequentially, based on the pattern of spore wall differentiation observed in all other Glomus species.
L1: An outer hyaline layer which was intact in the specimens examined, but which is reported to slough in descriptions of the species; 0.6-1.8 µm thick; producing a pinkish-red reaction in Melzer’s reagent; adherent to L2.
L2: A layer consisting of thin adherent sublayers (or laminae), light yellow-brown (0-0-40-0) in color; 4-9 µm thick. All sublayers form a dark red to slightly purplish red color in Melzer’s reagent.
L3: A thin flexible layer < 1.0 µm thick which is continuous with the innermost layer of the subtending hypha. It previously has been described as a “membranous wall”, but it clearly is a component part of the spore wall. It could not be determined from the specimens examined if this layer stains similarly to L2 in Melzer’s reagent.
| In PVLG | |
|---|---|
| In PVLG & Melzer's Reagent |
|---|
|
Subtending Hypha
SHAPE: Cylindrical to slightly flared (see photos above).
WIDTH: 8-10.2 µm
COMPOSITE WALL THICKNESS: < 1-2 µm.
WALL STRUCTURE: Two layers are clearly discernible which are continuous with L1 and L2 of the spore wall. L3 of the spore wall also may continue into the hypha, but more often appears to curve and form a septum-like structure.
OCCLUSION: Innermost layer of the spore wall (L3) appears to form a fragile septum. The laminate layer of the spore wall (L2) also may thicken, but rarely completely occludes the hyphal pore.
Notes
The intact spores photographed above were obtained from a culture provided by Terry McGonigle. However, the culture also contained numerous spores of R. intraradices which was very similar in size and color so that the two species were difficult to completely distinguish. Despite numerous attempts to establish cultures of R. fasciculatus from single and multiple spore inoculum, either no mycorrhizae were formed or the mycorrhizae was solely of R. intraradices. The broken spores were of a specimen provided by Jean Stutz which originated from the rhizosphere of Sporobolus wrightii.
More literature has been published using G. fasciculatum as the experimental organism than any other arbuscular fungal species (496 reports in our reference database), yet all cultures accessed in INVAM labelled as G. fasciculatum proved to group with the reference culture of R. intraradices. The photograph of spores aggregated around and within corn and tuliptree roots (Gerdemann, 1965) appears to be the archetype attributed to G. fasciculatum even though it is a behavior exhibited by other species as well. Since R. intraradices appears to be ubiquitous on at least 6 of the 7 major continents and has been widely cultures, it appears to be the most likely synonym for much of what has been published to date.
Descriptions of R. fasciculatus have been modified as more was learned about taxonomic characters and their interpretation (see Gerdemann, 1965; Gerdemann and Trappe, 1974; Hall and Abbott, 1984; and Walker and Koske, 1987). Clearly, the species is real, but it does not appear to be readily culturable (since one cannot be readily obtainable from ANY source). It is quite distinctive from other glomoid species to date in the intense dextrinoid reaction of the L2 (and possibly L3) layers of the spore wall to Melzer’s reagent. It also has a very thick spore wall relative to the diameter of the spore.
Links to Gene Sequences in Genbank
References
- Gerdemann, J. W. 1965. Vesicular-arbuscular mycorrhizae formed on maize and tuliptree by Endogone fasciculata. Mycologia 57: 562-575
- Gerdemann, J. W. and J. M. Trappe. 1974. The Endogonaceae in the Pacific Northwest 5: 1-76.
- Hall, I. R. and L. K. Abbott. 1984. Some Endogonaceae from southwestern Australia. Trans.Br.Mycol.Soc. 83: 203-208.
- Walker, C. and R. E. Koske. 1987. Taxonomic concepts in the Endogonaceae. IV. Glomus fasciculatum redescribed. Mycotaxon 30: 253-262.