Acaulospora laevis
(reference accession AU211)
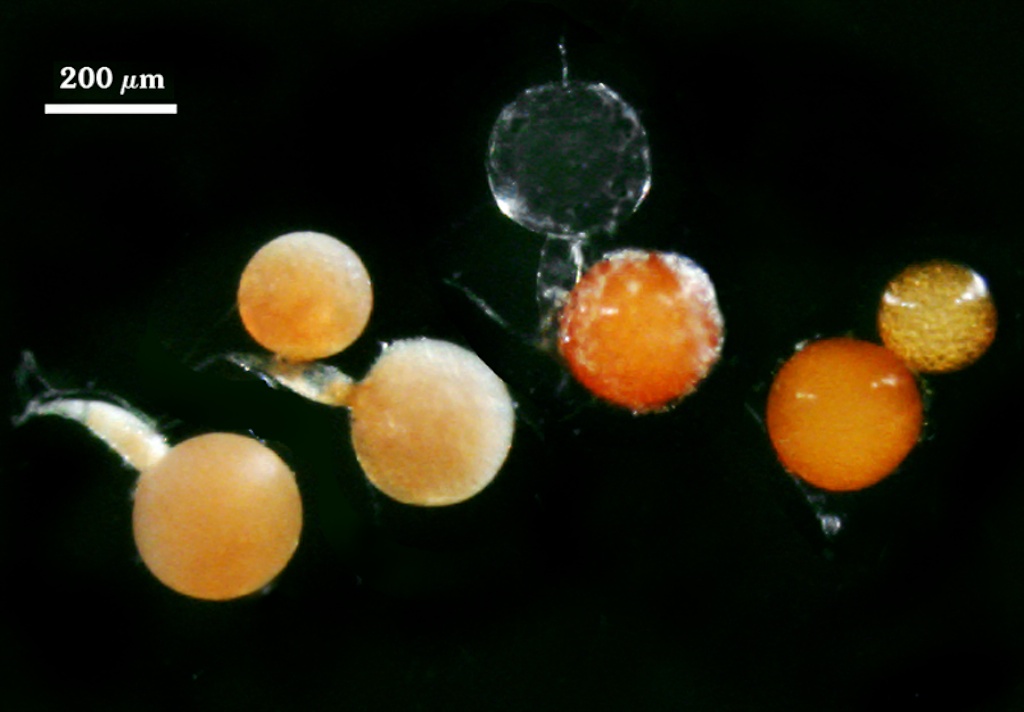
Whole Spores

 COLOR: Salmon (0-20-80-0) to orange-brown (0-60-100-10), most pale orange-brown (0-30-60-0).
COLOR: Salmon (0-20-80-0) to orange-brown (0-60-100-10), most pale orange-brown (0-30-60-0).
SHAPE: Globose, subglobose.
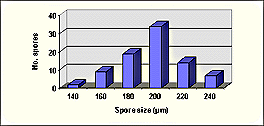
SIZE DISTRIBUTION: 140-240 µm, mean = 198 µm (n = 85).
Subcellular Structure of Spores
Spores consist of a three-layered spore wall and two bi-layered flexible germinal walls. Layers of the spore wall differentiate sequentiall (L1 to L3), after which each of the germinal walls differentiate sequentially (gw1 to gw2). This sequence is shown below, starting with the left-most photo.
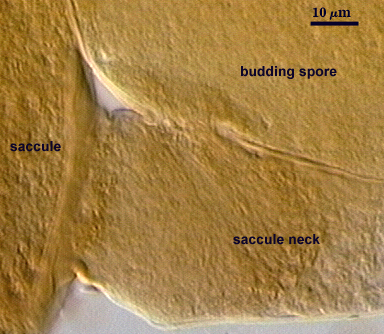
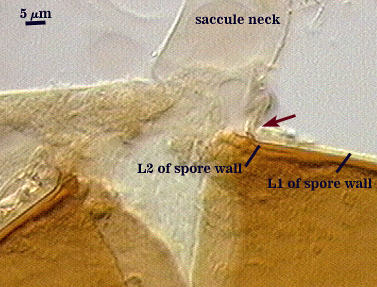
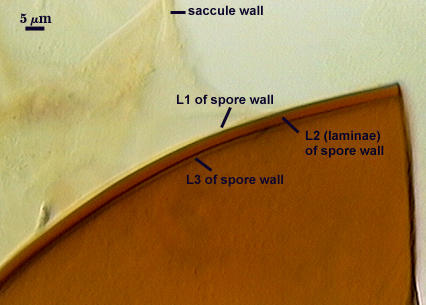
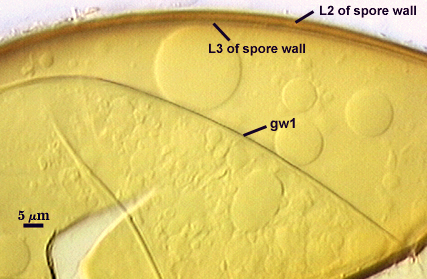
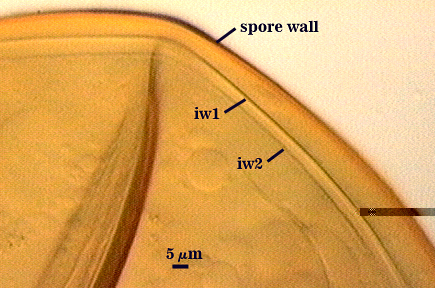
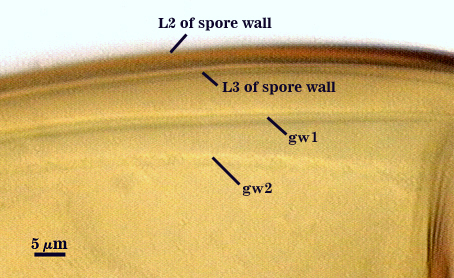
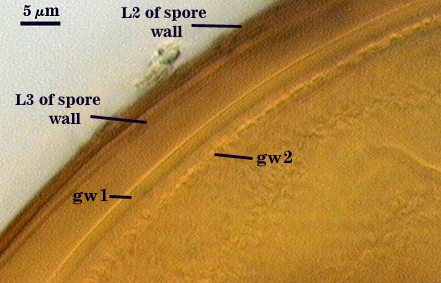
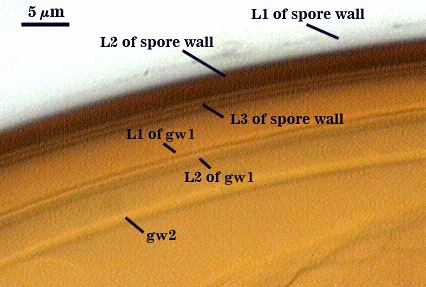
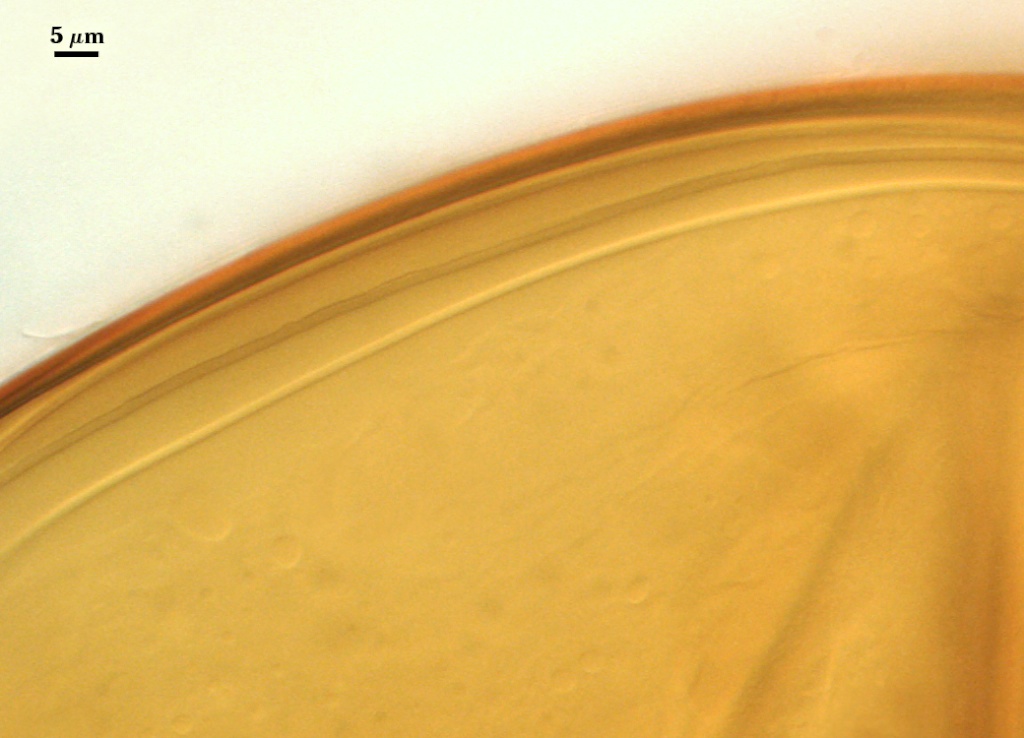
SPORE WALL: Three layers (L1, L2, and L3), the outer one continous with the wall of the neck of the parent sporiferous saccule and inner two synthesized as the spore expands from the saccule neck. L2 and L3 are formed sequentially during spore wall differentiation.
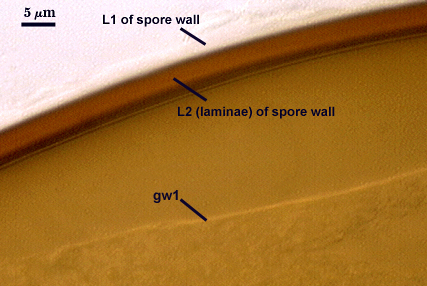
L1: Hyaline, smooth, 1.2-2.0 µm thick in juvenile spores; continuous with the wall of the saccule neck. It degrades or sloughs as the spore dehisces from the saccule neck.
L2: A layer consisting of very fine and often adherent sublayers, pale orange-brown (0-30-100-0) to darker orange-brown (0-20-100-0), 1.6-2.8 µm thick (mean of 1.9 µm). The surface of this layer is smooth, with no reaction in Melzer’s reagent.
L3: Another layer of lighter yellow-brown color (0-10-40-0), consisting of very fine tightly adherent sublayers, 1.2-1.6 µm thick (mean = 1.4 µm). No reaction in Melzer’s reagent.
| In PVLG | |
|---|---|
|
|
| In PVLG & Melzer’s reagent | |
|---|---|
|
|
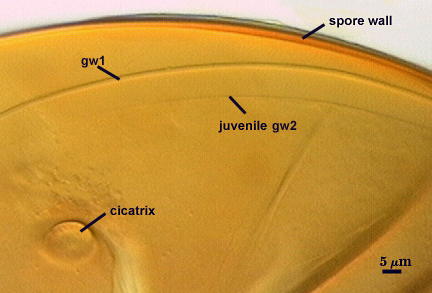
GERMINAL WALLS: Two flexible hyaline inner walls (gw1 and gw2) are present.
GW1: Two tightly adherent hyaline layers (L1 and L2) are formed. L1 is less than 0.5 µm thick. L2 is 1.0-1.8 µm thick.
GW2: Two tightly adherent hyaline layers (L1 and L2) are formed. L1 is 0.5-0.8 µm thick and coated on the surface with granular excresences (or “beads”) that tend to become dislodged and float away when pressure is applied to it. These “beads” are stabilized after preservation in formalin, but otherwise may be absent on mounted spores within a few weeks to months of storage. L2 is 0.5-1.0 µm thick and generally is nonreactive in Melzer’s reagent (on rare occasion producing a very pale pink reaction).
Cicatrix
Circular to ovoid scar indicating region of contact between spore and saccule neck during spore synthesis; 9-16 µm in diameter (mean = 12.8 µm).
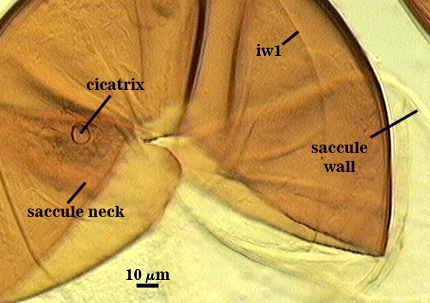
Sporiferous Saccule
COLOR: Hyaline
SHAPE: Mostly globose.
SIZE: 150-260 µm, mean = 228 µm.
SACCULE WALL: One layer, smooth surface, 2.8-4.9 µm thick (mean = 3.9 µm).
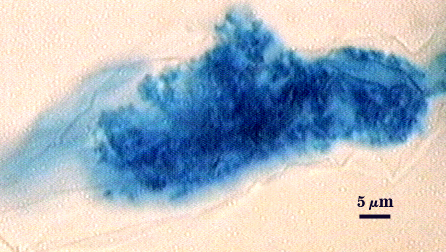
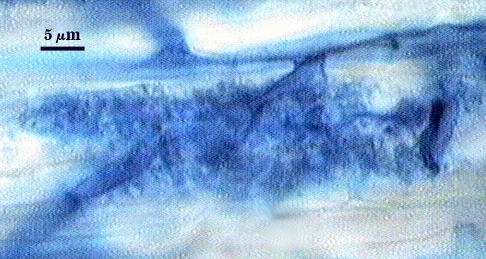
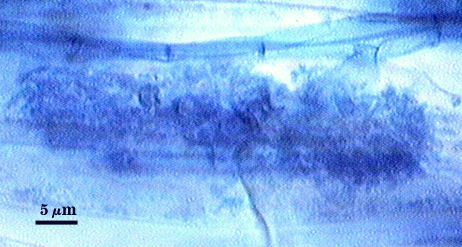
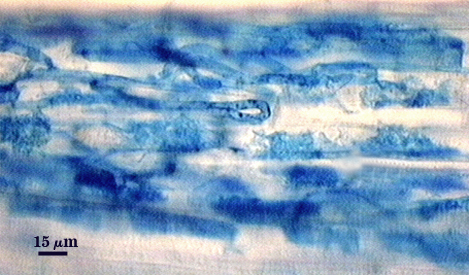
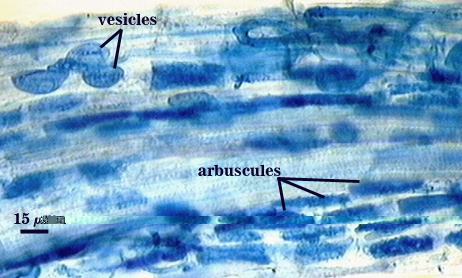
Mycorrhizae
Arbuscules and intraradical hyphae usually stain with variable intensity in trypan blue, ranging from pale to medium blue and moderate contrast. Staining is darkest at entry points, where hyphae form coils and usually are their widest (4-6 µm). “Foraging” hyphae that grow from these entry locations tend to be straighter, grow parallel to each other, and are thinner (2-5 µm). Oblong to irregular vesicles and attached hyphae are distributed mostly in clusters and usually near entry points.
| Arbuscules in cortical cells of corn | ||
|---|---|---|
|
|
|
| All mycorrhizal structures in corn | |
|---|---|
|
|
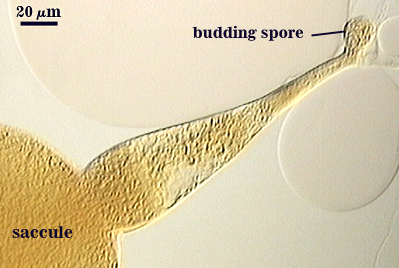
Germination
An ovoid “germination orb” forms on gw2, from which germ tubes form and penetrate through the spore wall. This orb is difficult to see except in older spores where contents have cleared with fusion of lipid globules in the spore lumen. It is present in the type specimen of the species.
Notes
Spores of this species mostly closely resemble those of A. koskei, which is of similar size, shape, and color, but which has a slightly different inner wall structure (L3 of the spore wall and L2 of gw2 producing a dextrinoid reaction in Melzer’s reagent).
This isolate was obtained from Lyn Abbott’s lab in Perth, Australia, and considerable work has been done with it in studying the life cycle of the species. Results of these studies suggest a long dormancy period (up to six months) before spores are infective as inoculum. Cultures grown at INVAM bear out this conclusion. Moreover, repeated culturing causes a sort of attentuation where infectivity drops along with sporulation. The remedy is to reseed an active culture to obtain regrowth of new roots, which seems to revive the fungus.