Rhizophagus manihotis
(reference accession FL879)
= Glomus manihotis
= Rhizophagus clarus
= Glomus clarum
Whole Spores
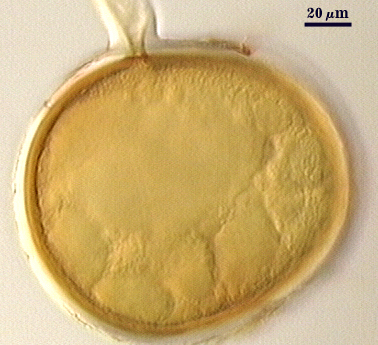
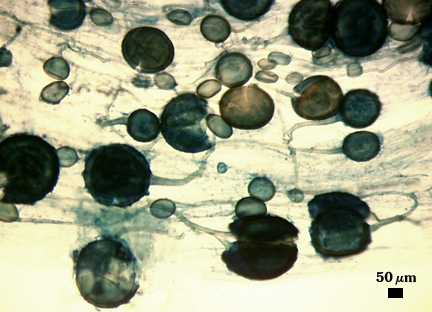
COLOR: A continuum from white to yellow-brown (0-10-60-0), with many pale yellow (0-0-20-0 to pale yellow brown (0-10-20-0).
SHAPE: Globose, subglobose, sometimes elliptical, oblong, or irregular (possibly spores formed in roots).
SIZE DISTRIBUTION: 100-260 µm, mean = 182 µm (n = 120).
Subcellular Structure of Spores
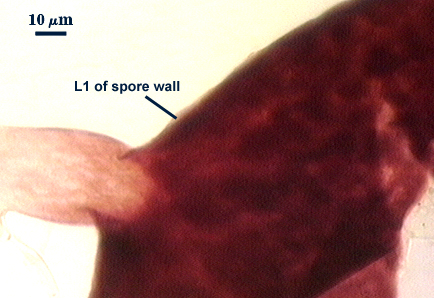
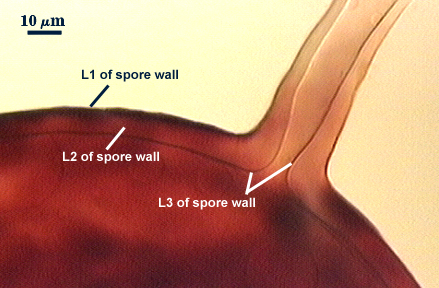
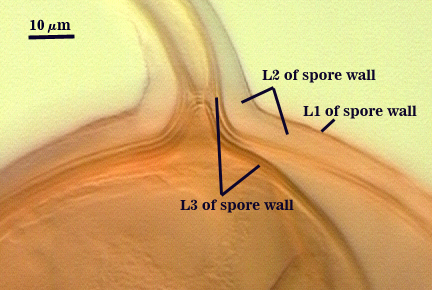
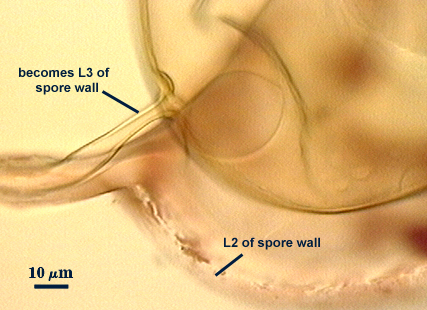
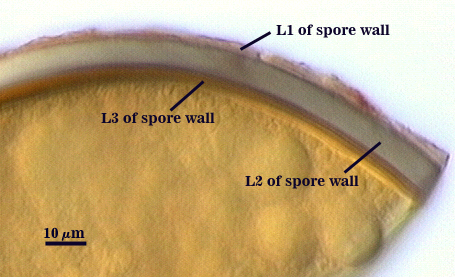
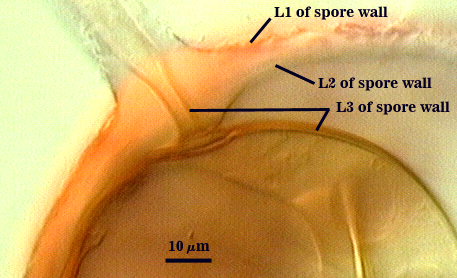
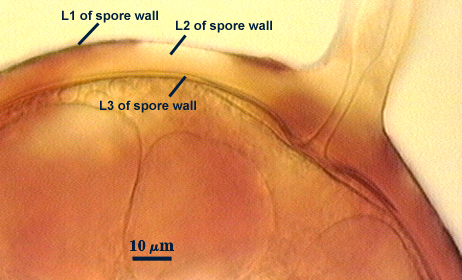
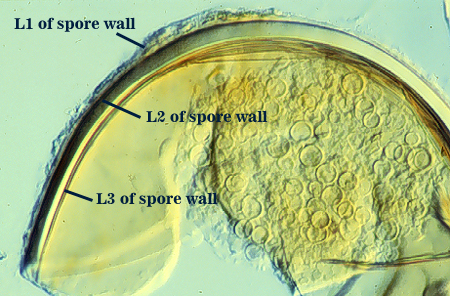
SPORE WALL: Three layers (L1, L2 and L3), all may be adherent but L3 often separates in mature spores with applied pressure. These layers form consecutively as the spore wall differentiates, with only L1 present in the spore and hyphal wall (developmental sequence from left to right in photos below).
| Developmental Sequence | ||||
|---|---|---|---|---|
L1: A hyaline mucilagenous layer, usually degrading and sloughing so that it often is absent on mature spores; 1.0-4.7 µm thick when even distributed on spore, but often expanding in patches up to 8.5 µm thick in very young spores; staining pinkish-red (20-80-20-0) to light purple (20-80-40-0) in Melzer’s reagent. This layer almost appears laminate in juvenile spores from striations in the thicker patches.
L2: A permanent hyaline layer; 9-14.5 µm thick (mean of 11.3 µm ); consisting of sublayers that are not brittle, but rather of a granular consistency that causes cracking and fragmentation of the spore wall; spores often appear “squashed” rather than cleanly broken (see photo at right). This layer is thicker (as much as 25%) in some regions so that the inner surface appears “wavy” and sometimes appears to have shallow knobs. The surface of this layer often appears flaky in PVLG and patchy in Melzer’s reagent due to uneven degradation of the outer layer (L1).
L3: Another permanent layer; usually consisting of 2-4 sublayers (or laminae); yellow (0-0-40-0) to dark yellow (0-10-60-0) in color; 1.2-3.5 µm thick (most near 2.5 µm). Sublayers usually are adherent, but they sometimes separate and thicken (up to 12 µm thick) at the juncture of the subtending hypha. The thicker this layer, the darker yellow the spore will appear under a stereomicroscope.
| In PVLG | |
|---|---|
| In PVLG & Melzer's reagent (1:1 v/v) | |
|---|---|
Subtending Hypha
SHAPE: Cylindrical to flared, occasionally slightly constricted (see photos above).
WIDTH: 18-24 µm (mean = 22.1 µm).
HYPHAL WALL: Three layers (L1, L2 and L3) near the spore, with L1 thinning to invisibility within 5 µm of the spore surface; composite thickness of 6.4-10.6 µm near the spore (mean of 8.7 µm), tapering to less than 3 µm with only L3 visible. L1 is mucilagenous and the only layer present in juvenile subtending hyphae, at which time the hyphal wall is < 1.0 µm thick. L2 is laminate and is continuous with L2 of the spore wall. It appears to arise de novo at the same time it is synthesized in early spore expansion; 4.8-11.2 µm thick near the spore at maturity. L3 is visible as a very thin layer (usually < 0.8 µm thick, occasionally up to 1.6 µm) and pale yellow (0-0-10-0). In some white spores, this layer cannot be seen..
OCCLUSION: Innermost sublayer of L3 of the spore wall bridges the pore, although often it is so thin that the pore appears to be open (see photos above). In some spores, the spore wall thickens and almost occludes the pore; a plug is formed with rare frequency.
Mycorrhizae
Identical to that of Rhizophagus clarus, including prolific sporulation in roots.
| Rhyzophagus clarus | |
|---|---|
|
|
Notes
Schenck et al. (1984) based their distinction of R. manihotis from R. clarum mainly on color (the former being yellow and the latter white to very pale yellow). They further justified the distinction by a greater tendency of the subtending hypha of R. manihot to break off near the spore. This isolate is derived from the cultotype (CIAT C-1-1) and has been maintained through three pot culture generations in INVAM and thus provides the means to compare living cultures of R. manihotis and R. clarus grown simultaneously. In this comparison, the distinction between the two species becomes blurred to nonexistent. The cultotype produces a large proportion of dark yellow spores, but white to pale yellow spores also are produced. Some isolates in INVAM produce spores that are consistently white, but they tend to be at the small end of the size distribution, others are highly variable in color (changing with pot culture generation), and still others are mostly yellow to yellow-brown. This continuum eliminates any morphological distinction between R. clarus and R. manihotis.
Developmental studies show that color differences are attributable totally to the thickness of L3 of the spore wall, which varies considerably within all isolates examined. The same range of variation was evident in type specimens of both R. clarus and R. manihotis (see photo at right of latter). This variation then is a produce of heterochrony, whereby timing in termination of synthesis of sublayers in L3 differs from spore to spore. The more sublayers that are formed, the darker the layer and the yellower the spore.
rDNA and beta-tubulin gene sequences place both species together in the same clade, providing more definitive evidence they are conspecific. Probably the only convincing evidence of divergence between R. clarus and R. manihotis is in loci coding for malate dehydrogenase, as determined by gel electrophoresis (Rosendahl et al., 1994). However, these differences were correctly interpreted as allelic variation which measures differences between “individuals” forming mycorrhiza at different locations rather than species-level divergence. Such a pattern would certainly be expected among geographically isolated populations, irregardless whether they are sexual or asexual (the latter being the case for these fungi).
References
- Rosendahl, S., J. C. Dodd, and C. Walker. 1994. Taxonomy and phylogeny of Glomales. pp. 1-12. In: Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems. S. Gianinazzi and H. Schüepp (eds.) Birkhäuser Verlag, Basel, Switzerland.
- Schenck, N.C., J. L. Spain, E. Sieverding, and R.H. Howeler. 1984. Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76: 685-699.