Funneliformis mosseae
(reference accession UK115)
| Juvenile spores | Mature spores, with peridium |
|---|---|
 |  |
Sporocarps
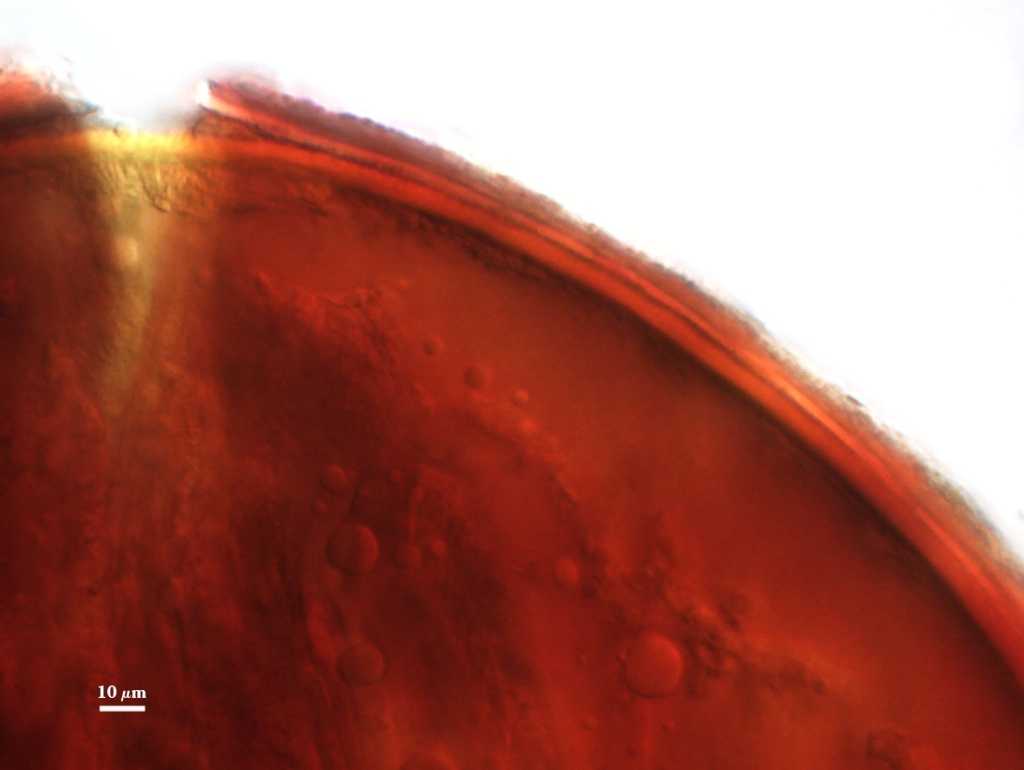
Some accessions in pot culture produce spores in clusters of 2-10 surrounded by a tight peridium (see photo above). This behavior varies not only among accessions, but between culture generations of a single accession and even among spores produced in the same pot.
Clusters are yellow-brown (0-40-100-10) to brown (20-40-100/-0)
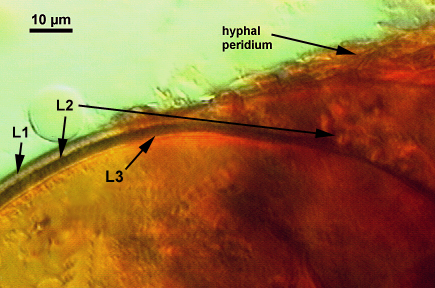
The peridium surrounding these spores is 10-38 µm thick, with robust hyphae (8-18 µm wide with walls 1.6-3.5 µm thick) mixed with many finer branching hyphae (2-5 µm wide, walls < 1 µm thick). The peridium does not alter spore wall structure, and appears to be a labile character (usually being lost after several successive cultures).
Whole Spores
 COLOR: Straw (0-5-20-0) to dark orange-brown
COLOR: Straw (0-5-20-0) to dark orange-brown
(0-30-100-10), but a majority are yellow-brown (0-10-60-0)
SHAPE: Globose to subglobose, some irregular
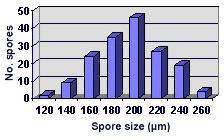
SIZE DISTRIBUTION:100-260 µm, mean = 195 µm (n = 166)
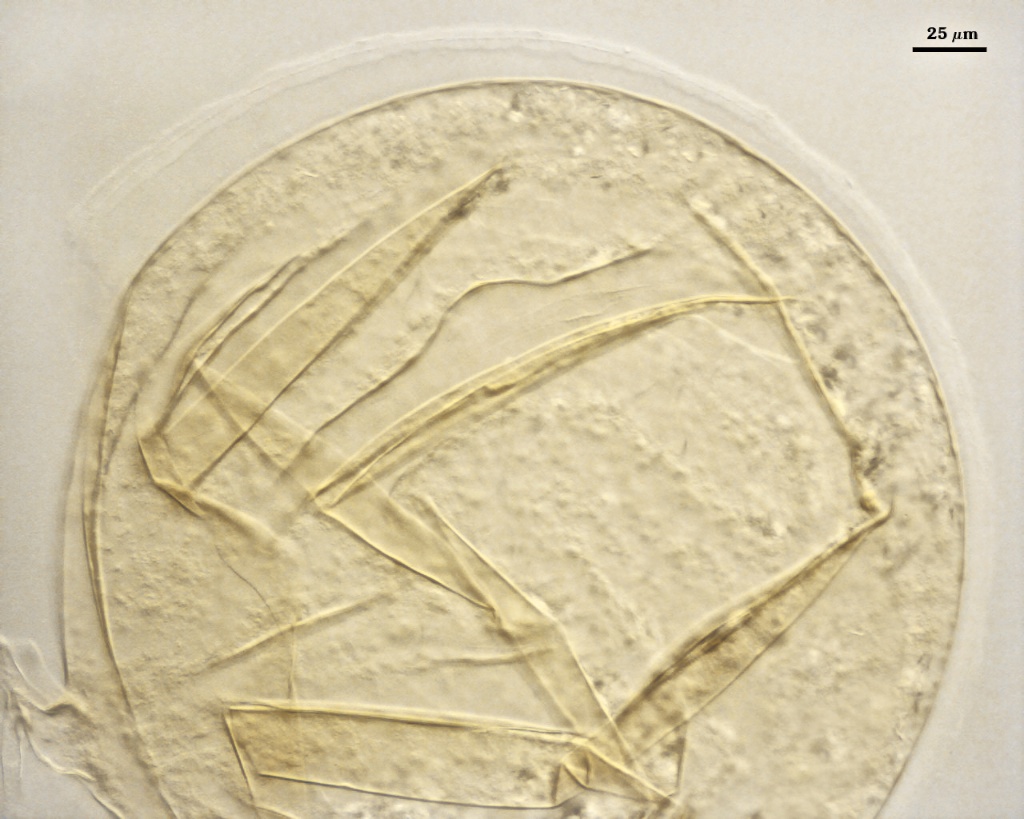
Subcellular Structure of Spores
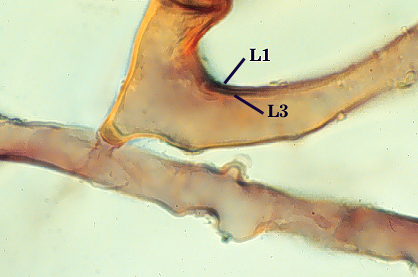
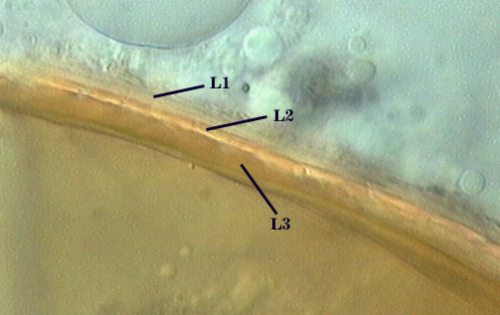
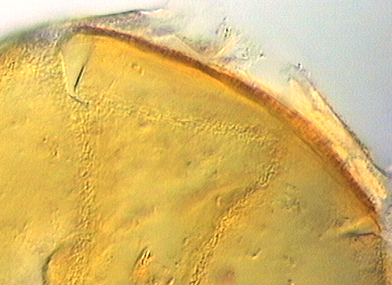
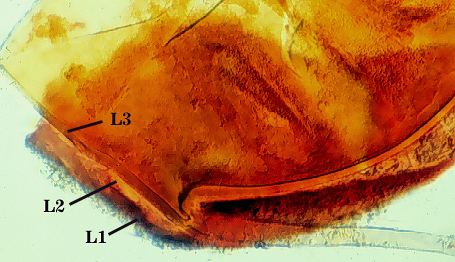
SPORE WALL: Three layers (L1, L2 and L3) that form consecutively as the spore wall differentiates (sequence below, from left to right). The outer two often slough to varying degrees in mature or older spores (especially those in field soils). Spores of some isolates (e.g. those from Rothamsted, England), cluster together within a compact peridium. This character, however, is of little taxonomic value because it is unstable. In low organic matter soils (like the sand:soil pot culture medium used by INVAM), accessions that consist mostly of peridium-constrained aggregates form single spores with no peridium after 1-2 successive propagation cycles on sudangrass.
| Mosseae Spore | ||
|---|---|---|
 |  | 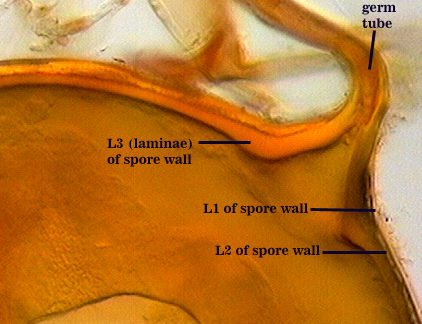 |
L1: Hyaline, mucilagenous, 1.4-2.5 µm thick (mean = 2.1 µm); staining pinkish-red in Melzer’s reagent (0-20-20-0 to 0-60-20-0); often degrading and then forming a sloughing granular layer, sloughing in mature spores, appearing granular in advanced stages of degradation; 2.5-3.5 m thick when intact on juvenile spores.
L2: Hyaline, 0.8-1.6 µm thick (mean = 1.2 µm), very refractile when viewed with differential contrast optics, generally rigid and fracturing into sliver-like fragments observed when this layer separates from L3. It must be attached firmly to the underlying laminae because small irregularly-shaped and shallow pits appear as parts of this layer break away with application of pressure. Not reactive in Melzer’s reagent. This layer appears to be quite variable in its appearance among spores of the same accession and also between accessions. It is uncertain whether this layer just isn’t being synthesized or it is just not detectable.
L3: A layer consisting of yellow brown (0-10-80-0) to pale orange-brown (0-20-80-0) sublayers (or laminae), 3.2-6.4 µm thick (mean of 4.7 µm); minute depressions cover the surface with separation of L1 and L2.
| In PVLG | |
|---|---|
 |  |
| In PVLG and Melzer's Reagent (1:1 v/v) | |
|---|---|
 | 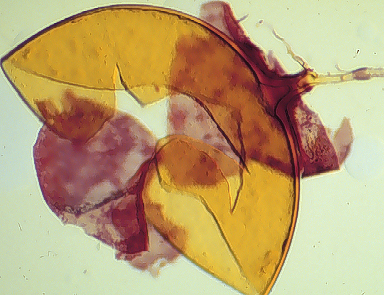 |
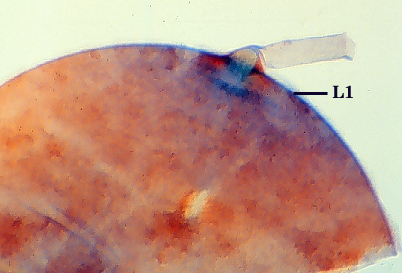
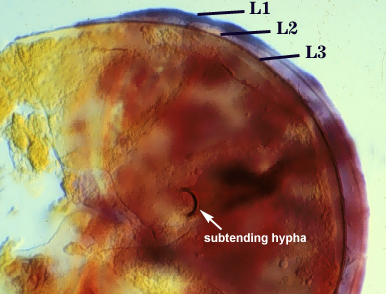
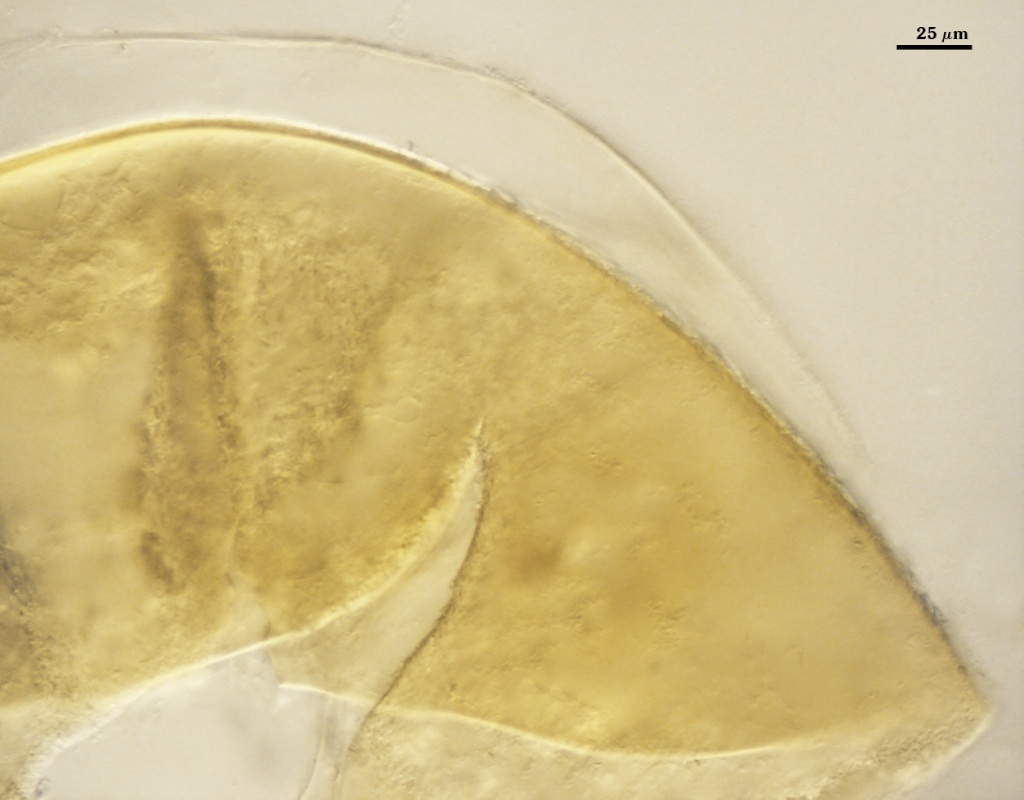
Subtending Hypha
SHAPE: Flared to funnel-shaped (see photos above).
WIDTH: 16-32 µm (mean = 24 µm).
HYPHAL WALL: Only two layers are observed, the outer continuous with L1 and the inner continuous with L3 of the spore wall, together 2.4-4.8 µm in thickness near the spore. The outer layer thins to 1.2-1.6 µm within 50 µm of the spore. Rarely present on the hypha of mature spores. The inner layer is concolorous with L3 of the spore wall, with sublayers adherent.
OCCLUSION: A recurved septum forms 0-30 µm from the point of attachment to the spore (see photos above).
Germination
ermG tube emerges from the lumen of the subtending hypha, originating from the recurved septum. These germ tubes branch extensively after emergence from the hypha. Sporocarps are most dramatic in producing numerous infective hyphae.
Mycorrhizae
Intraradical hyphae, arbuscules and vesicles stain darkly in trypan blue. Early in mycorrhizal development, external hyphae branch and form numerous thin-walled hyaline extramatrical “vesicles” with a very simple wall structure. They decline as colonization and sporulation increases. Intraradical hyphae may coil near entry points, with hyphae 3-6 µm wide, but then generally grow parallel to each other and to the root axis. The latter “foraging” hyphae are 1.5-3 µm wide. Vesicle production is very sporadic, with most isolated from each other. Abundance does not often increase that much as a mycorrhiza ages. Arbuscules form from successively thinner hyphal branches (see middle arbuscule below for best detail) from a trunk hypha that generally is 3-4 µm wide. Late in mycorrhizal development, roots contain mostly intraradical hyphae and sporadic arbuscules and vesicles.
| Arbuscules in corn root cortical cells | ||
|---|---|---|
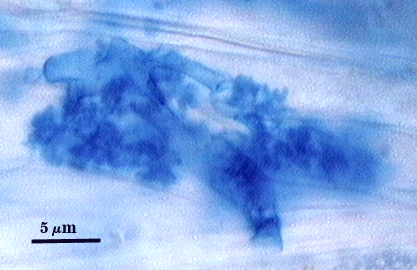 | 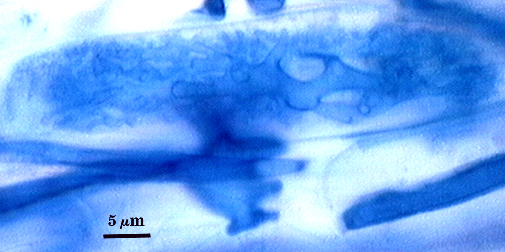 | 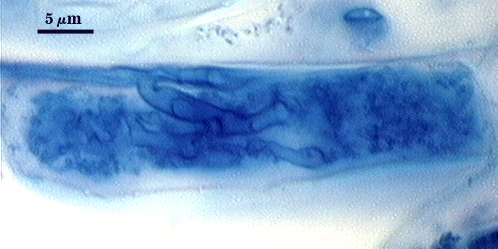 |
| Mycorrhizal structures in corn | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
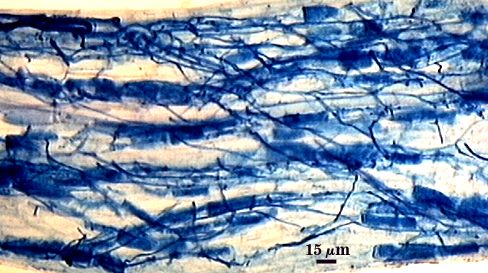 | 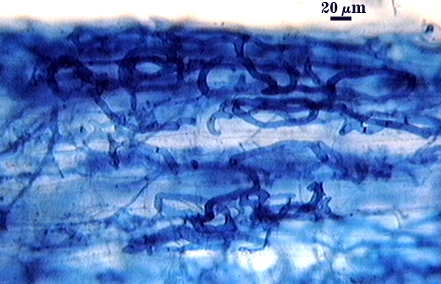 |  | |||||||||||||||||||||||||||||
Notes
The middle layer of the spore wall (L2) does not appear to be synthesized consistently in all spores of a population and the frequency of occurrence varies among culture generations of one isolate and among isolates of the species. It may form in all spores, but be so thin in some that it is not detected.
All available evidence indicates that F. monosporum is synonymous with F. mosseae. Another species often misidentified as F. mosseae is Funneliformis caledonium. However, spore wall structure of F. caledonium has four layers, each with distinct phenotypes.
As mentioned above, peridium synthesis and sporocarp formation are not conserved traits in this species. Some isolates from the United Kingdom produced abundant sporocarps in the first culture cycle, but then reverted to single spores with smooth surfaces by the third culture cycle. Sporocarps sometimes are formed, but with much less frequency. Of the > 80 isolates in the collection, in no instance did the behavior of forming spore singly change to sporocarp formation. It is likely that the pot culture environment and/or the duration of culture maintenance are not conducive to induction of a peridium and sporocarps.
The images below can be uploaded into your brower by clicking on the thumbnail or can be downloaded to your computer by clicking on the link below each image. Please do not use these images for other than personal use without expressed permission from INVAM.
High Resolution Images | |
|---|---|
 |  |
 |  |
 |  |