Acaulospora rugosa
(ex-type WV949)
Whole Spores

 COLOR: Subhyaline (0-0-10-0) to pale yellow brown (0-10-40-0), with the former color most abundant. Color is fairly uniform among spores in a given population.
COLOR: Subhyaline (0-0-10-0) to pale yellow brown (0-10-40-0), with the former color most abundant. Color is fairly uniform among spores in a given population.
SHAPE: Mostly globose, subglobose, occasionally irregular.
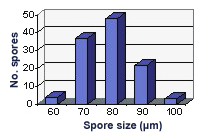
SIZE DISTRIBUTION: 60-100 µm, mean = 74 µm (n = 120)
Subcellular Structure of Spores
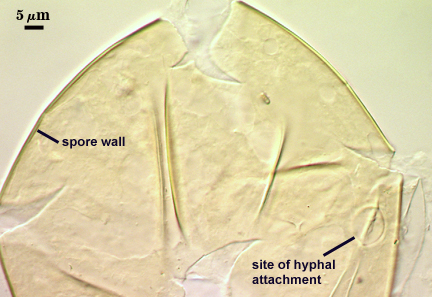
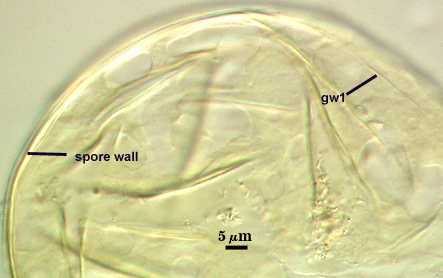
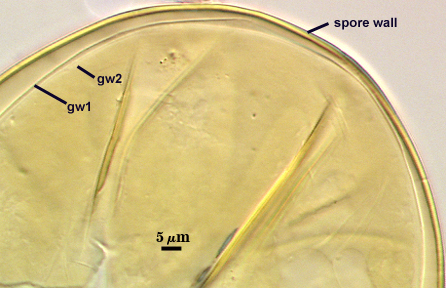
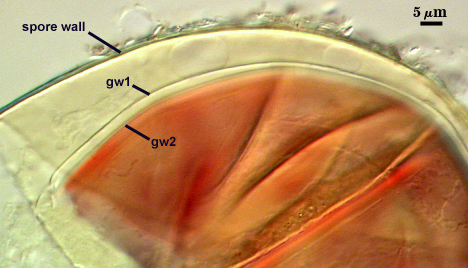
The developmental sequence in formation of (1) layers in the spore wall, (2) the first flexible germinal wall and (3) the second germinal wall are shown in the photographs below (from left to right). The mature spore (with full plasticity and dark red-purple reaction of L2 in gw2) is shown in the second set of photographs below.
| Developmental Sequence | |
|---|---|
 |  |
 |  |
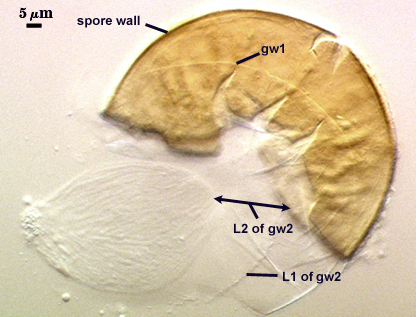
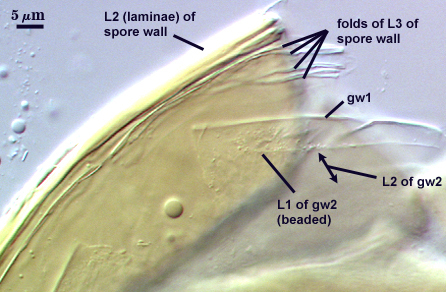
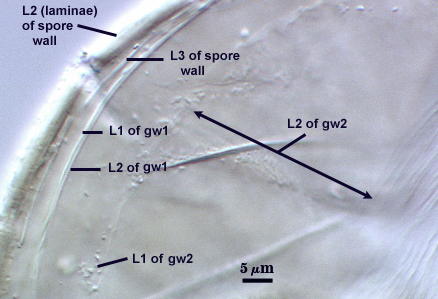
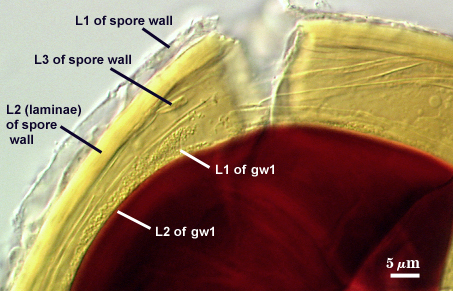
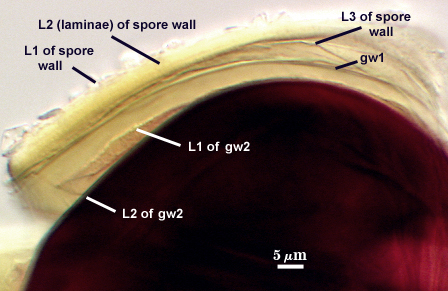
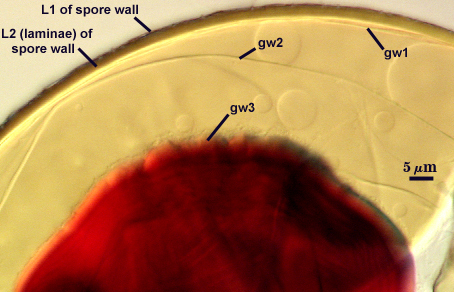
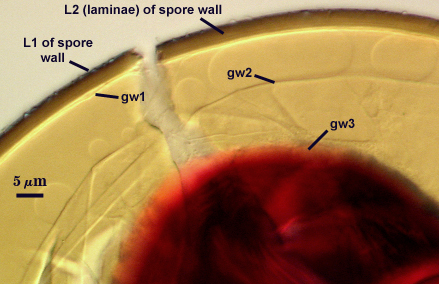
SPORE WALL: Three layers (L1, L2, and L3), the outer one continous with the wall of the neck of the parent sporiferous saccule and the latter two synthesized with origination of a spore.
L1: Hyaline, thin (0.5-1.2 µm), and so flexible that it often produces extensive wrinkles in PVLG when present. When this layer does not slough, it produces numerous folds on the spore surface and appears “rugose”. This layer has been defined as a “membranous wall”, but it clearly originates as an extension of the hyphal wall of the sporiferous saccule from which the spore wall arises and therefore is considered a component layer of the spore wall.
L2: A layer consisting of very fine and adherent sublayers (or laminae), pale yellow (0-0-20-0), 2.0-3.2 µm (mean = 2.4 µm) thick. Surface is smooth if the outer layer has sloughed, which often occurs after sucrose-density centrifugation and washing (susceptibility of this layer to slough also appears to be isolate-dependent). At maturity, the pore between spore and saccule neck is closed by continuous sublayers of this layer to resemble an “endospore”.
L3: A thin layer, less than 1 µm thick. It often is adherent to the spore wall in which case it cannot be detected. Only with some degree of separation from the spore wall (spores vigorously broken) can it be seen. Even then, it may produce folds that cause it to appear more numerous than truly is the case (see photos below).
| Spores mounted in PVLG | ||
|---|---|---|
|
|
|
| Spores in PVLG + Melzer’s reagent (1:1 v/v). | |
|---|---|
|
|
|
|
GERMINAL WALLS: Two flexible hyaline inner walls (gw1 and gw2), with iw1 sometimes obscured if it does not separate cleanly from the spore wall, but gw2 always separates and is easily identified. Folds produced by iw1 often can be deceiving and erroneously suggest additional flexible inner walls.
GW1: Two layers, with a composite thickness of 1-1.8 µm thick. This wall often does not separate readily from the spore wall or produce folds that make interpretation difficult. Each layer of this wall is extremely thin (usually < 0.6 µm), and both are of near-equal thickness. When both layers are adherent (as they often are), this wall appears to be monolayered. However, enough spores are found in all isolates which show two distinct thin layers with differential contrast optics.
GW2: Two adherent layers, which are readily distinguishable in Melzer’s reagent because of contrasting reactions. L1 is hyaline, 0.5-1.2 µm thick, with granular excresences (or “beads”) that tend to become dislodged and float away with applied pressure. These “beads” are stabilized after preservation in formalin, but otherwise may be absent on mounted spores within a few months of storage. This layer is most discretely observable when the “beads” are absent because it does not produce more than a pale pink color reaction and thus contrasts nicely from the inner L2 layer. L2 is hyaline and plastic and in conventional terminology has been described as “amorphous”. It ranges from 8-15 µm thick in PVLG-based mountants, depending on amount of pressure applied to it while breaking the spore, and stains red-purple (40-80-20-0) to dark red-purple (60-80-60-0) in Melzer’s reagent. The intensity of the reaction in Melzer’s reagent decreases considerably in old stored spores, parasitized spores, or spores preserved in lactophenol, formalin, or sodium azide. In spores preserved for longer than 60 days in formalin, gw2 loses its plasticity (and considerable color in Melzer’s reactions) and measures 1.4-3 µm thick.
Cicatrix
Scar indicating region of contact between spore and saccule neck during spore synthesis; very narrow lip (< 1 µm) circumscribing the scar, circular to oval-shaped, 7-12 µm at widest (mean = 9.2 µm ).
Sporiferous Saccule
COLOR: Hyaline.
SHAPE: Mostly globose to subglobose, occasionally irregular.
SIZE: 80-110 µm, mean = 93 µm.
SACCULE WALL: One layer, smooth surface, 0.8-1.2 µm thick.
DISTANCE FROM SACCULE TO SPORE: 60-130 µm.
Notes
Spores rarely found with saccules attached, even in early pot culture harvests. Spore differentiation thus is thought to occur very quickly. All available evidence indicates this species should be merged with Acaulospora morrowiae. Morton (1986) failed to detect L3 of the spore wall or the bilayered organization of gw1 in his description of A. rugosa and he placed too much weight on presence of L1 of the spore wall (which proved to slough more in some isolates than others, with all other properties being equal), He also was not aware at the time that A. morrowiae had been imprecisely described with too narrow a range in spore color and nonrecognition of the plasticity of the inner layer (L2) of gw2 (Schenck et al., 1984). With the acquisition of isolates from Florida identified by Norm Schenck as A. morrowiae, it quickly became clear that isolates of both species were indistinguishable and overlapped completely in all continous characters and contained exactly the same discrete characters. It is interesting that the cultotype of A. rugosa deposited in INVAM when at Florida had notes associated with it questioning whether it was A. morrowiae.
Reference
- Morton, J. B. 1986. Three new species of Acaulospora (Endogonaceae) from high aluminum, low pH soils in West Virginia. Mycologia 78: 641-648.
- Schenck, N. C., J. L. Spain, E. Sieverding, and R. H. Howeler. 1984. Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76: 685-699.