Sclerocystis sinuosum
(reference accession MD126)
We have confirmed this species as genus Sclerocystis. Further website updates to follow.
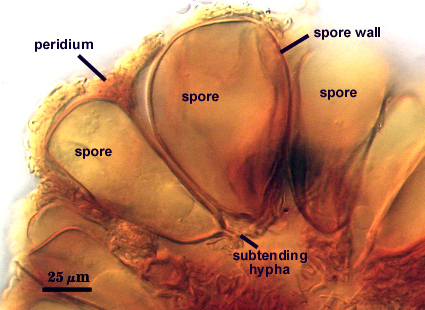
Sporocarps
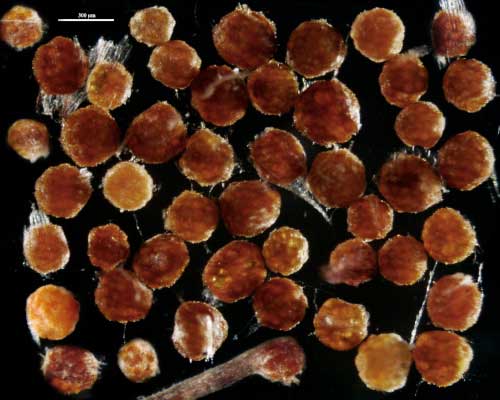
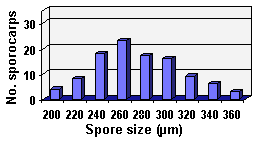 COLOR: Orange (0-40-80-0) when immature, becoming orange-brown (0-60-100-0) to dark orange-brown (20-60-100-0) when mature. Sporocarps often form within roots in pot culture after only three months using sudangrass as the host plant.
COLOR: Orange (0-40-80-0) when immature, becoming orange-brown (0-60-100-0) to dark orange-brown (20-60-100-0) when mature. Sporocarps often form within roots in pot culture after only three months using sudangrass as the host plant.
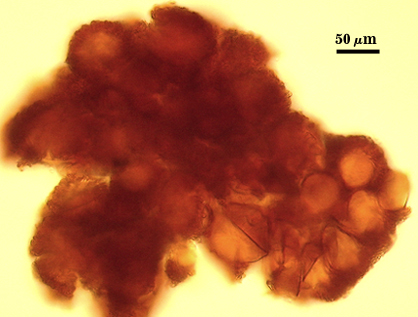
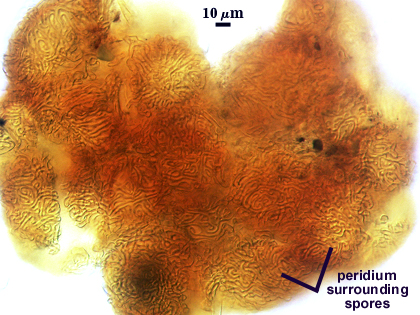
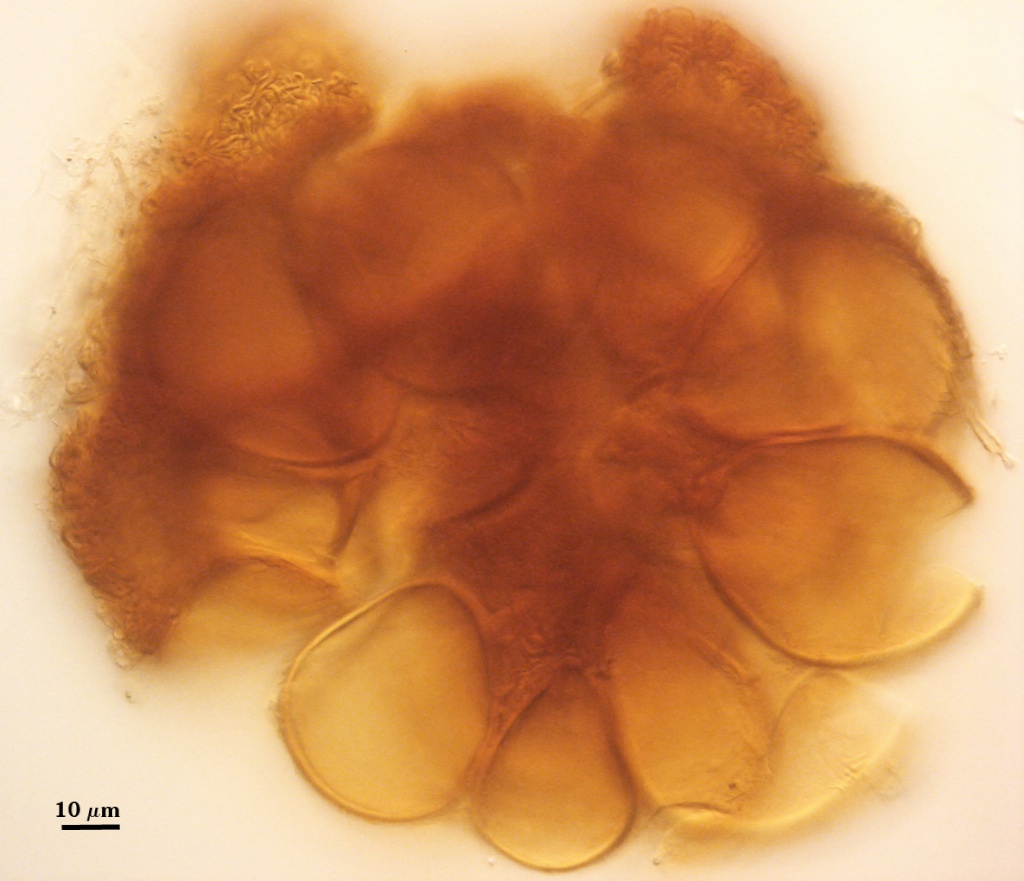
SHAPE: Globose, subglobose, occasionally pulvinate; irregular surface due to protruding spores covered by a dense peridium.
SIZE DISTRIBUTION: 200-360 µm; mean = 273 µm (n = 104)
Peridum
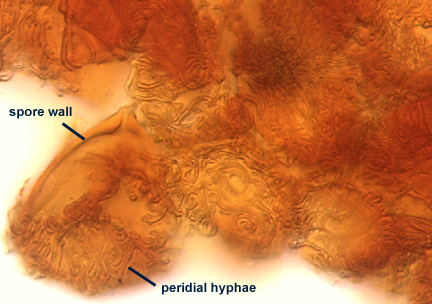
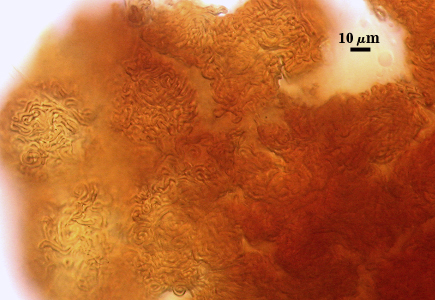
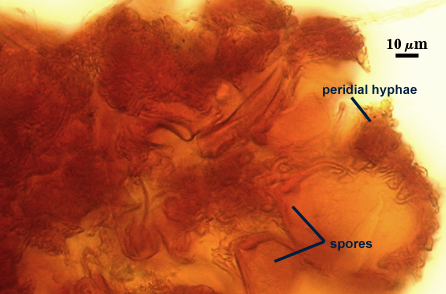
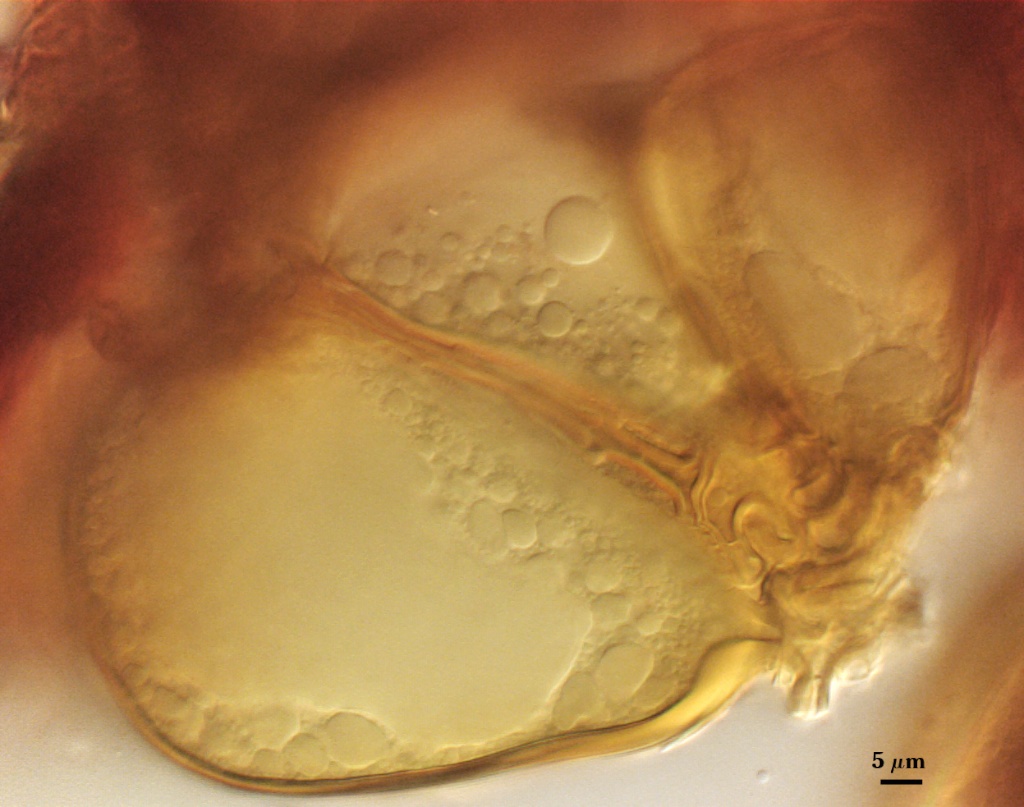
A dense layer of tightly interwoven hyphae, 9-19 µm thick, covering all spores to keep them tightly packed. To see spores clearly, this peridium must be cut with a scalpel before sporocarps are mounted on a slide. Hyphae are 2.5-7 µm in diameter with walls 0.5-1 µm thick.
| In PVLG | |
|---|---|
| In Melzer's reagent | ||
|---|---|---|
| ||
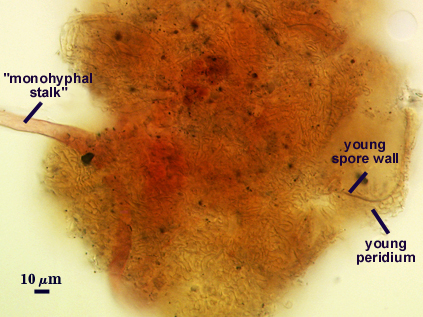
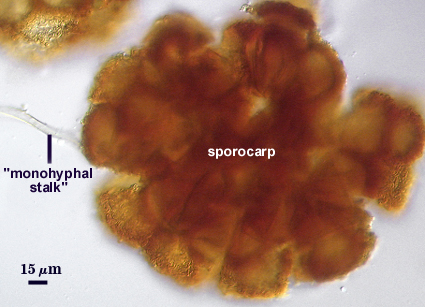
The peridium appears to develop from a “monohyphal stalk” (Wu, 1993) and is a tightly packed network of hyphae even in immature sporocarps.
| Young sporocarp (in Melzer's) | Mature sporocarp (in PVLG) |
|---|---|
|
|
Whole Spores
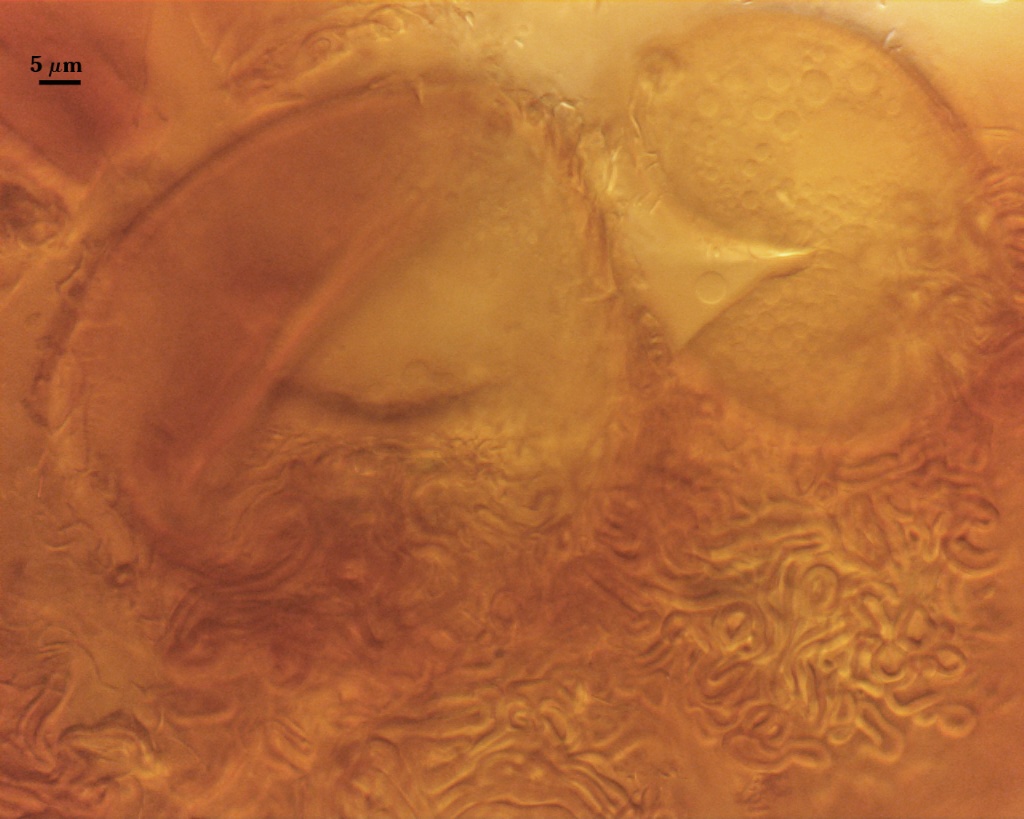
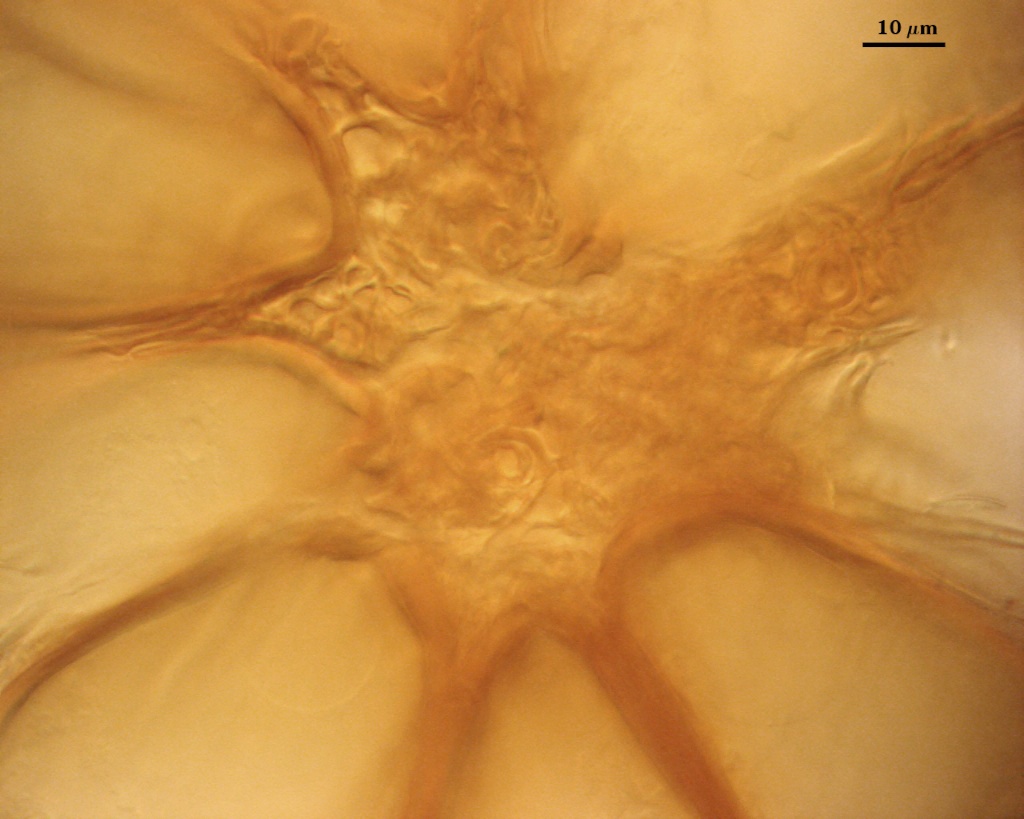
Spores usually were clavate, but sometimes obovate to elliptical; organized in a “single layer from a central plexus of hyphae” (Gerdemann and Bakshi, 1976); orange-brown in color; 28-63×50-95 µm (w x l) in size.
| In Melzer’s reagent | ||
|---|---|---|
|
|
|
Subcellular Structure of Spores
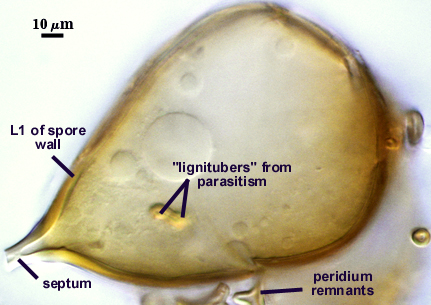
SPORE WALL: One layer (L1) surrounded by dense peridial hyphae (see photos above).
L1: Pale orange-brown (0-10-60-0 to 0-20-80-0) sublayers (or laminae) that always are adherent. Thickness varies considerably on a single spore, 1.5-6 µm, being thickest at base. In some older spores, the apex also will thicken more than the sides of elliptical or clavate spores.
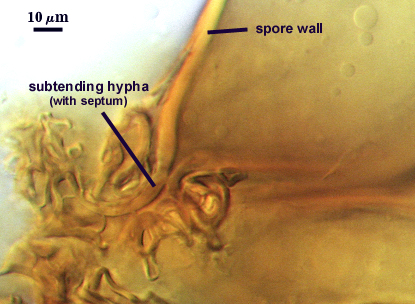
Subtending Hypha
SHAPE: Cylindrical to slightly flared, although shape at the spore often is hard to detect because of sometimes profuse side branching connected to the central plexus hyphae (see photos above).
WIDTH: 4-8 µm
WALL STRUCTURE: A single layer continuous with the spore wall layer, concolorous, 2-4.2 µm thick.
OCCLUSION: Usually a thin septum, but occasionally by thickening of the spore wall sublayers (see photos above).
Germination
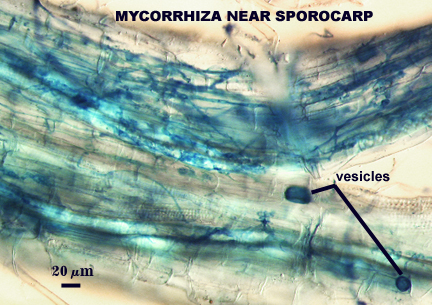
Not observed, but apparently germination occurs frequently from the central hyphal plexus of sporocarps sampled by others (Almeida and Schenck, 1990; Wu, 1993a). Reports indicate germ tubes produce extramatrical vesicle-like structures.
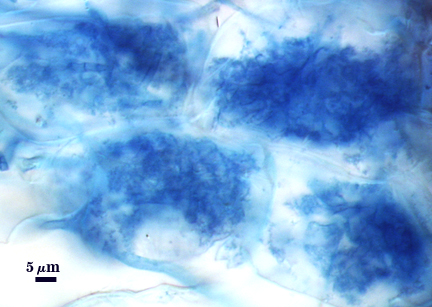
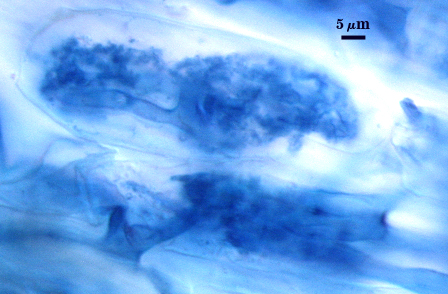
Mycorrhizae
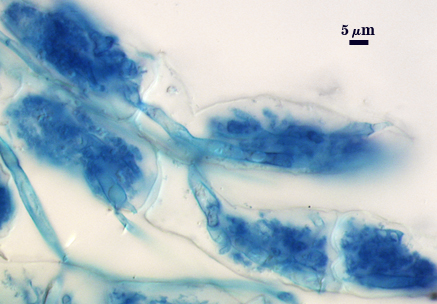
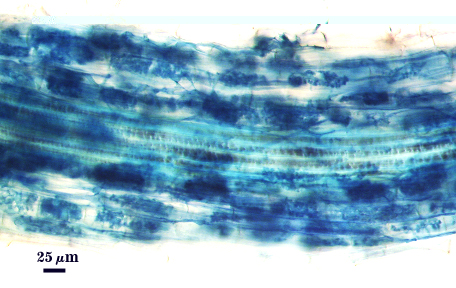
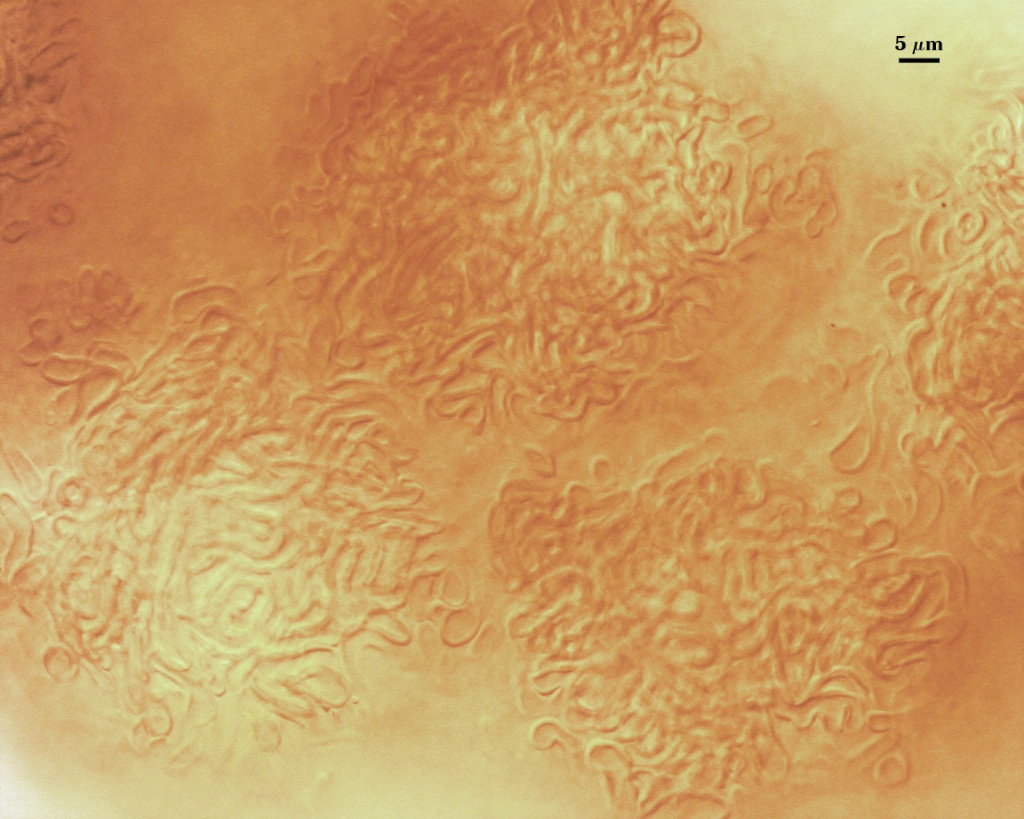
Arbuscules and hyphae in cortical cells of sorghum, sudangrass, corn and red clover roots stain darkly in trypan blue. Numerous vesicles (or spores) often form near entry points along with the arbuscule-hyphal network. It is uncertain as the extent intraradical vesicles are still able to further differentiate into spores, because they can co-occur in roots.
| Arbuscules in 90-day-old corn plants | ||
|---|---|---|
| Mycorrhizae in 90-day-old corn | |
|---|---|
| Sporocarps linked by hyphae to roots | |
|---|---|
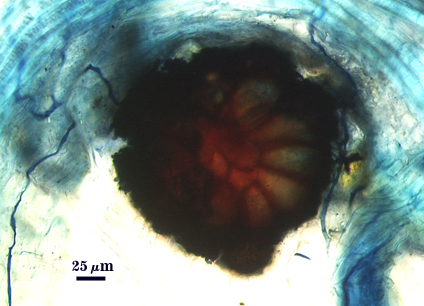 | 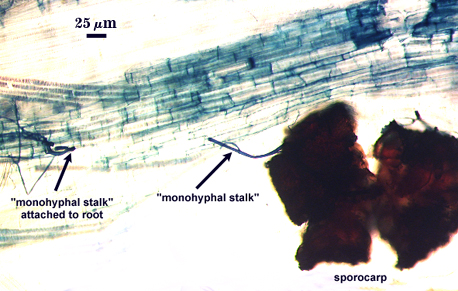 |
Notes
This species reportedly was established in monospecific pot culture by Almeida and Schenck (1990), but the one culture of this fungus tranferred from Florida to West Virginia contained only spores of Paraglomus occultum. It took almost two years to establish a culture from sporocarps (many were heavily parasitized) and this reference culture is maintained constantly by reseeding at six month intervals. Sporocarps and spores closely resemble those of type specimens and those pictured by Almeida and Schenck (1990) and Wu (1993a, b).
Stages of sporocarp development are difficult to establish because a dense aggregate of sinuous hyphae form almost immediately on the “monohyphal stalk”. Wu (1993a) attempts to compare development amongst related sporocarpic species, but his interpretations were indirect and based only on field-collected specimens. Interestingly, the spores appear to form concurrently from the central plexus, after which the spore walls differentiate and thicken together as well.
Early SSU data placed this species in Glomus when it was viewed as a single monophyletic clade (Redecker et al., 2000). Schüßler, and Walker (2010) erected a number of genera to recognize distinct clades within Glomus and one of these was Sclerocystis. They placed this species in that clade (monospecific at that time) which they deemed distinct from other glomoid clades . LSU sequences we have obtained don’t show this separation and instead places this species within the Rhizophagus clade. A consensus classification (Redecker et al., 2013) maintained this species in Sclerocystis for the benefit of compromise, but we take the liberty to disagree here and include it in Rhizophagus. Part of the difficulty in interpretation of this distinction is the small sample size—there just aren’t enough sporocarpic “Sclerocystis” species (sensu lato) to clarify this issue yet.
Our rationale adds morphological considerations, which we consider just as important as sequence data in phylogenetic analysis. First, S. sinuosum shares a life history trait of sporulating within and outside roots with other Rhizophagus species. Second, the sporocarpic habit is a convergent trait, most evident in other genera such as Redeckera and Diversispora and so it is not a trait unique to Sclerocystis as perceived in “the old days.” This argument parallels that of convergence in position of the spore in relation to a sporiferous saccule, obviating Entrophospora as a phylogenetically valid genus. This has been a productive culture for more than 15 years, and so this inoculum likely will behave well in other environments.
The images below can be uploaded into your browser by clicking on the thumbnail or can be downloaded to your computer by clicking on the link below each image. Please do not use these images for other than personal use without expressed permission from INVAM.
High Resolution Images | |
|---|---|
 |  |
 |  |
 |  |
References
- Almeida, R. T. and N. C. Schenck. 1990. A revision of the genus Sclerocystis (Glomaceae, Glomales). Mycologia 82:703-714.
- Gerdemann, J. W. and B. K. Bakshi. 1976. Endogonaceae of India: two new species. Trans. Br. Mycol. Soc. 66:340-343.
- Redecker, D., J. B. Morton, and T. D. Bruns. 2000. Molecular phylogeny of Glomus sinuosum and Sclerocystis coremioides places both taxa firmly in Glomus. Mycologia 92:282-285.
- Redecker, D., A. Schüßler, H. Stockinger, S. Stürmer, J. Morton, and C. Walker. 2013. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza doi:10.1007/s00572-013-0486-y.
- Schüßler, A and C. Walker C. 2010. The Glomeromycota: a species list with new families. Electronic copy available online at Glomeromycota PHYLOGENY.
- Wu, C-G. 1993a. Glomales of Taiwan: III. a comparative study of spore ontogeny in Sclerocystis (Glomaceae, Glomales). Mycotaxon 47:25-39.
- Wu, C-G. 1993b. Glomales of Taiwan: IV. A monograph of Sclerocystis (Glomaceae). Mycotaxon 47:327-349.