Diversispora spurca
(reference accession HA567)
Whole Spores
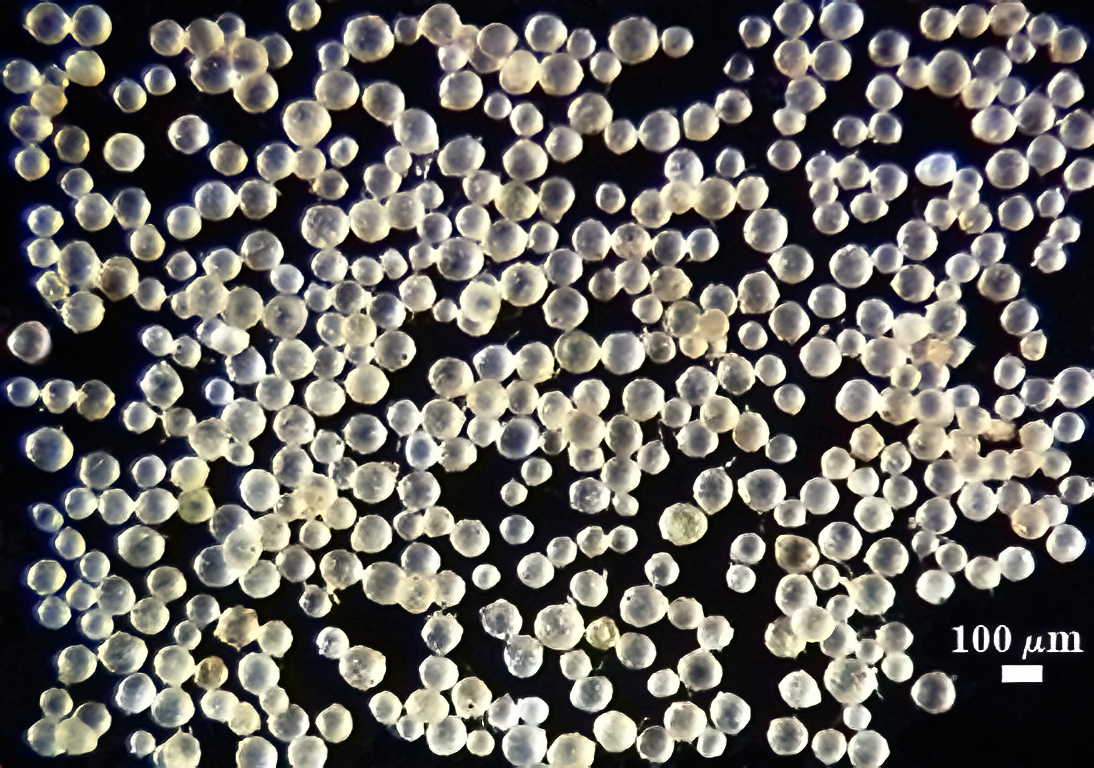
 COLOR: Subhyaline (0-0-5-0) to pale yellow brown (0-5-20-0), generally with a somewhat dull surface due to adherent debris.
COLOR: Subhyaline (0-0-5-0) to pale yellow brown (0-5-20-0), generally with a somewhat dull surface due to adherent debris.
SHAPE: Globose, subglobose.
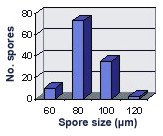
SIZE DISTRIBUTION: 60-120 µm, mean = 85 µm (n = 120).
Subcellular Structure of Spores
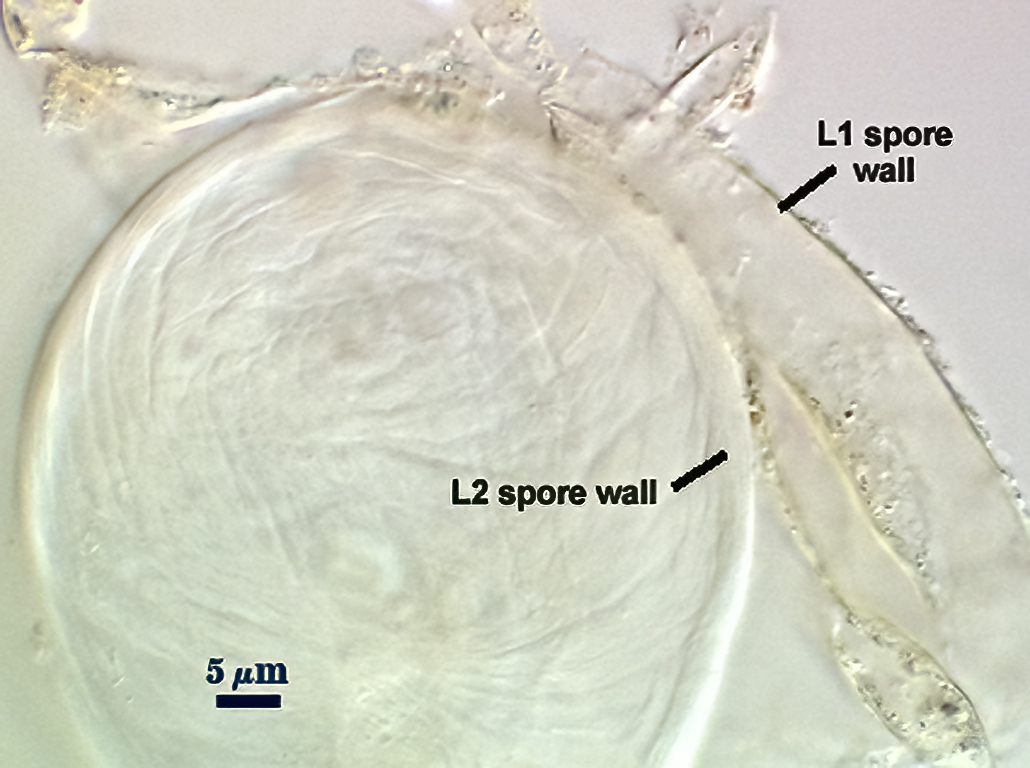
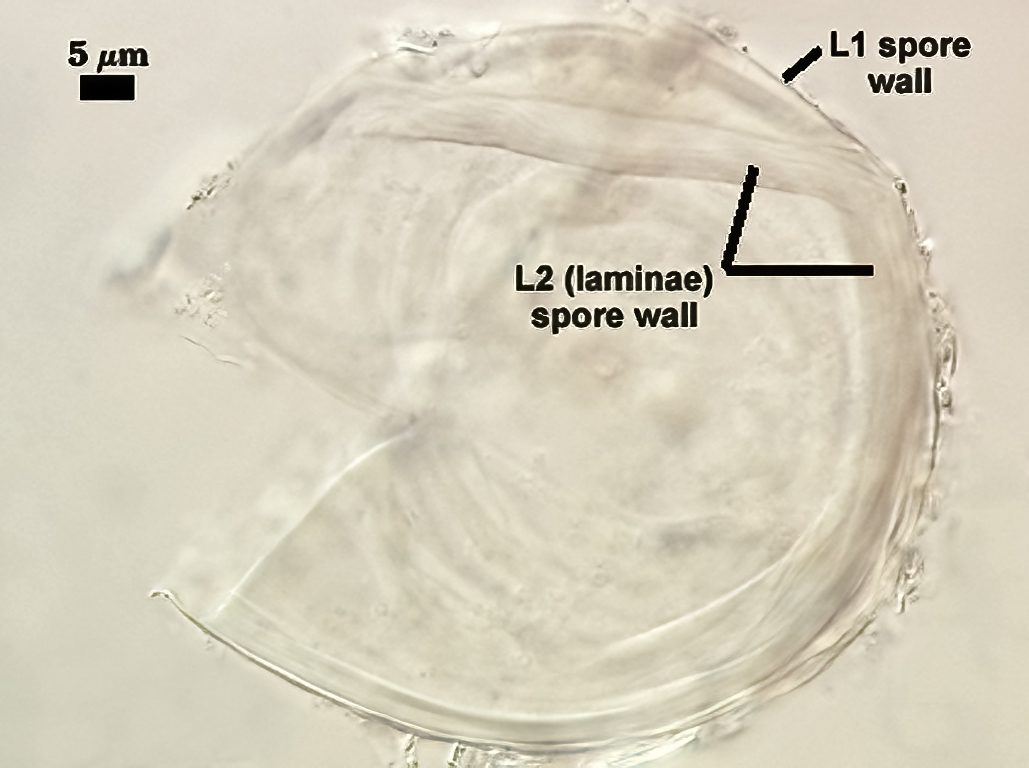
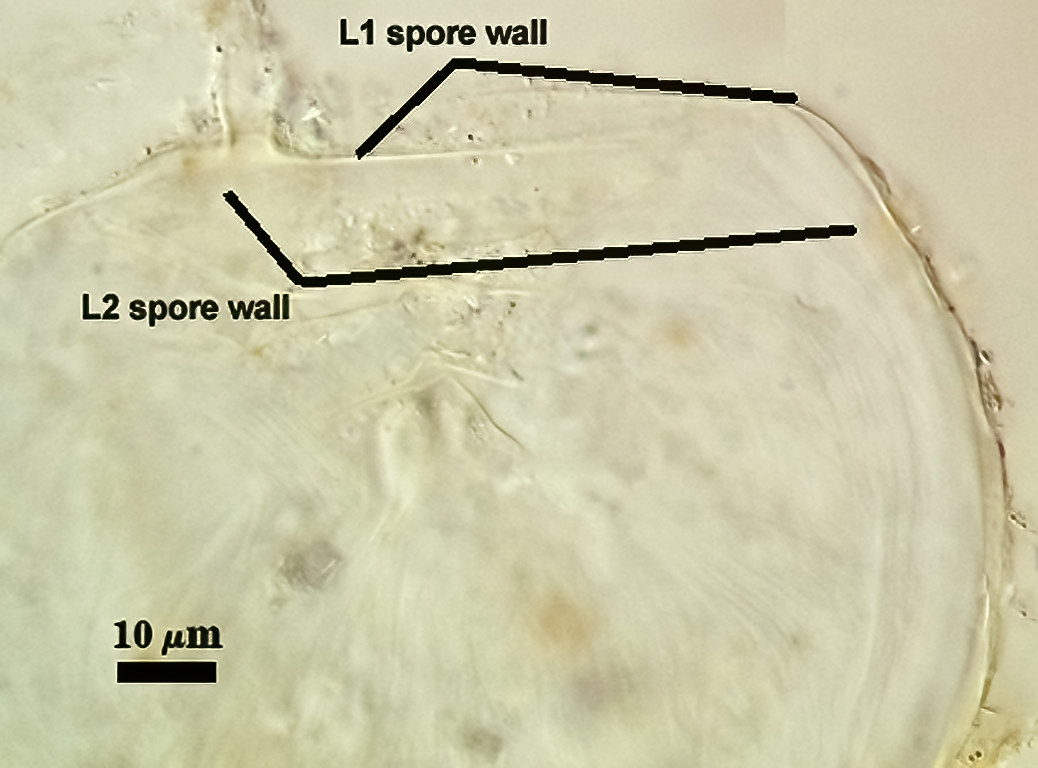
SPORE WALL: Two layers (L1 and L2), L1 often separating from L2 in vigorously crushed spores.
| In PVLG | ||
|---|---|---|
 |  |  |
| In PVLG and Melzer's reagent (1:1 v/v) | ||
|---|---|---|
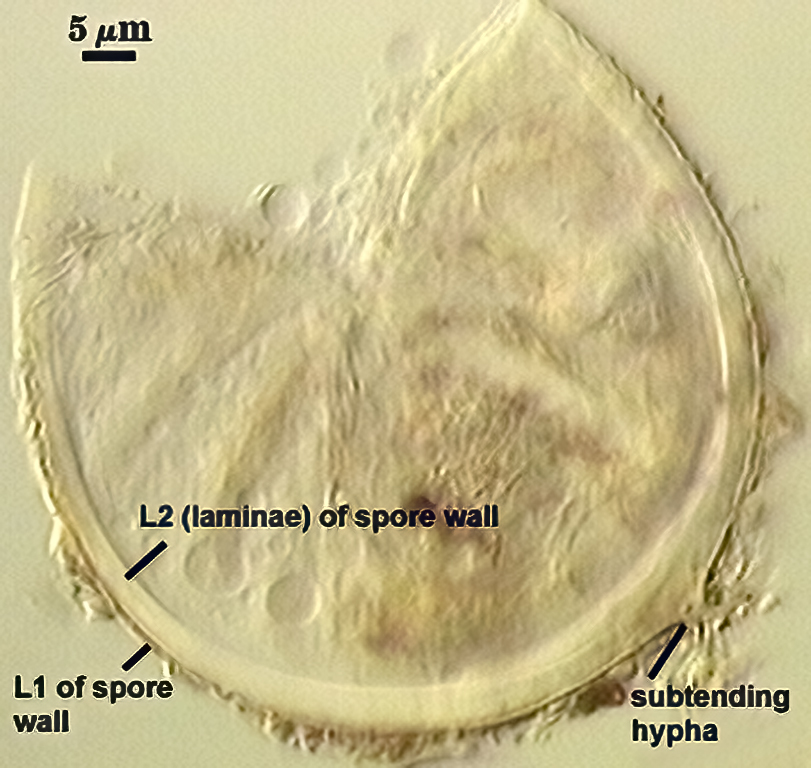 | 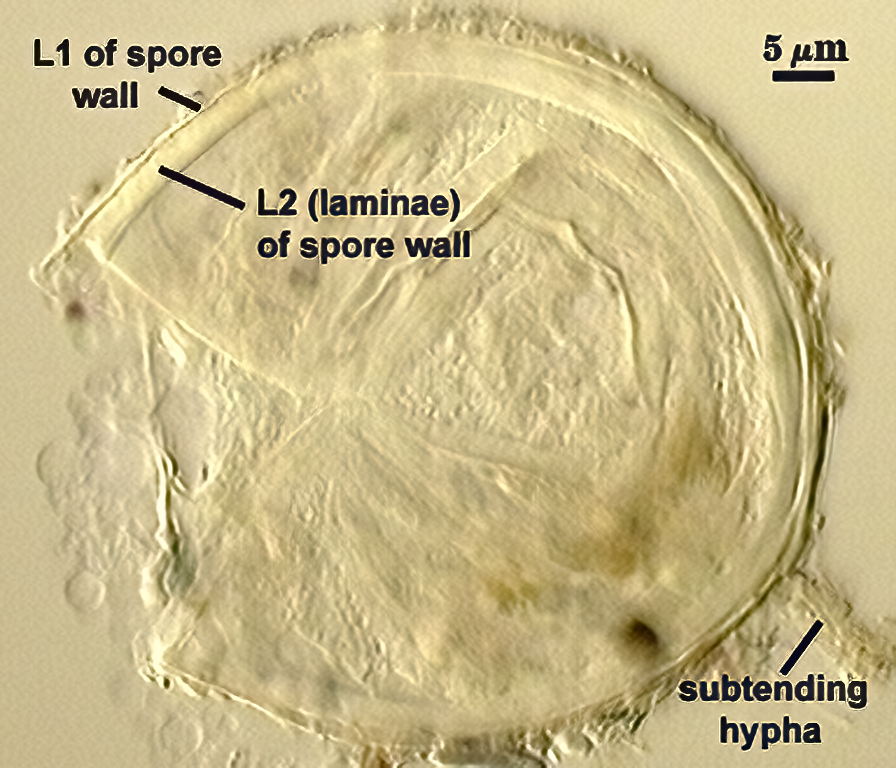 | 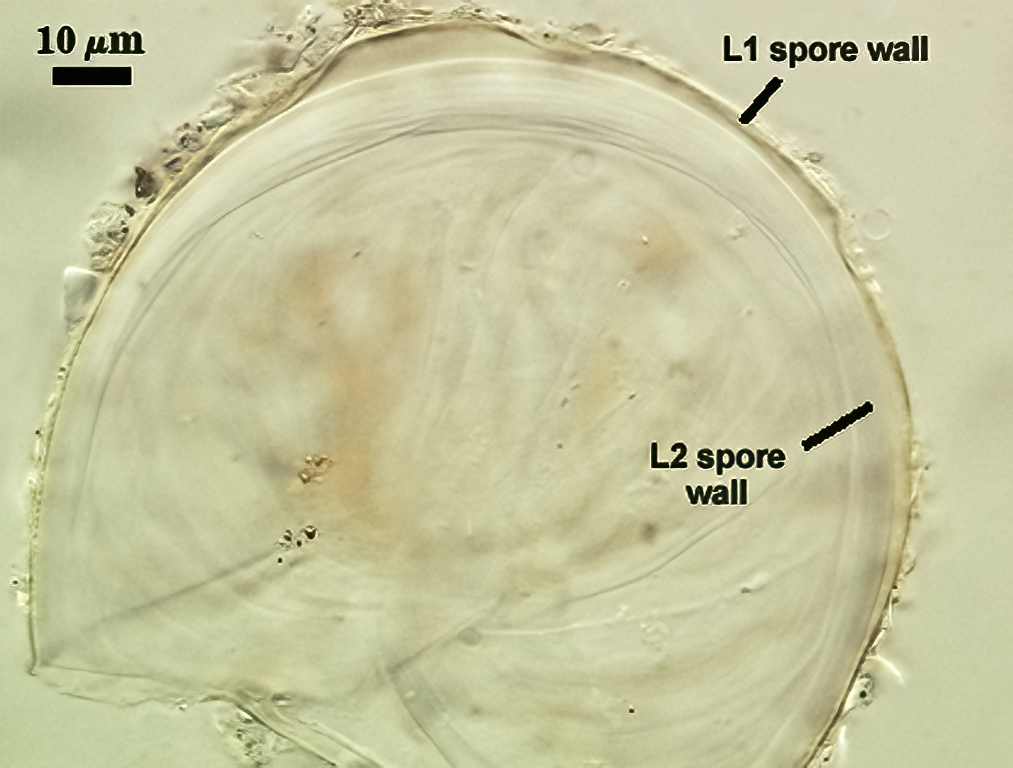 |
L1: Hyaline to pale yellow-brown (0-50-20-0) in transmitted light, 0.4-1.2 µm thick (usually between 0.6-0.7 µm); thin enough to often wrinkle and produce many folds on the spore surface leading to partial or complete separation from the inner laminate layer (L2). Often coated with adhering organic debris.
L2: A layer consisting of thin hyaline to subhyaline sublayers (or laminae), 1.4-4.2 µm thick (mean of 2.8 µm). This layer exhibits considerable plasticity and will spread with applied location pressure, to the extent it can measure as much as 12 µm thick in some spores where the spore wall has been “flattened” by applied pressure. The innermost sublayer(s) often produces numerous fine wrinkles that may be misinterpreted as a flexible inner layer associated with the spore wall (or a “membranous wall” by conventional terminology).
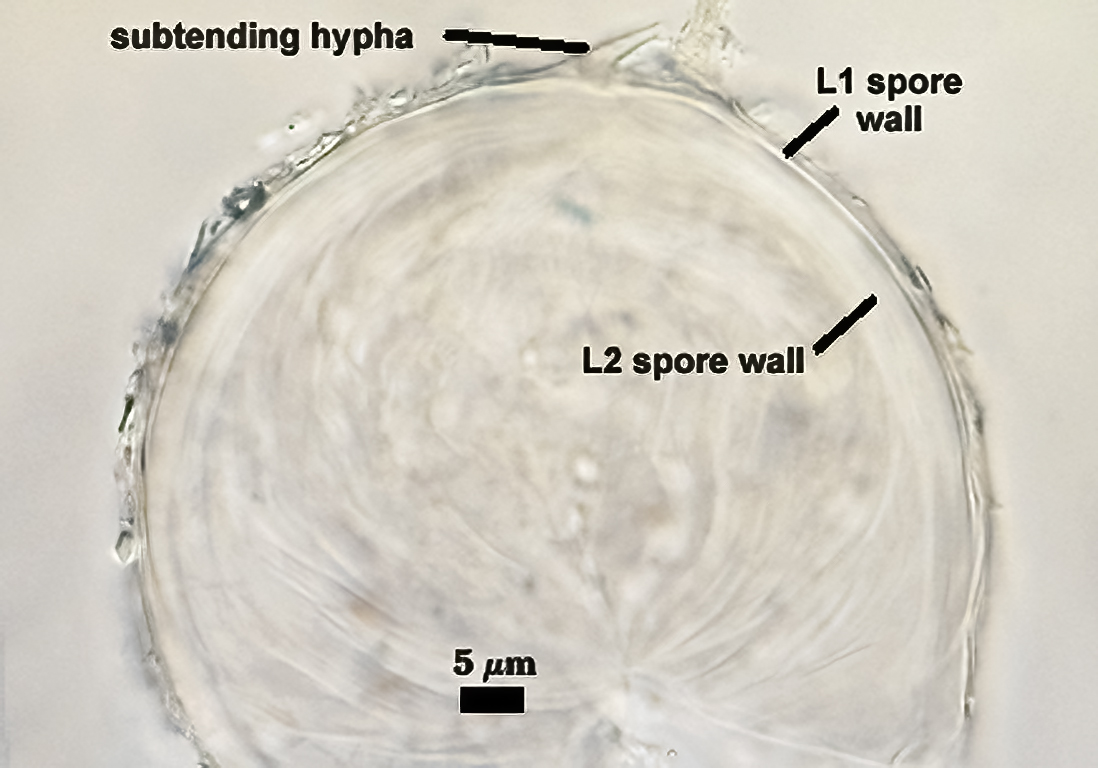
Subtending Hypha
SHAPE: Cylindrical to slightly flared.
WIDTH: 4.8-7.9 µm (mean = 6.2 µm).
WALL STRUCTURE: Beyond the region of attachment to the spore, only a single layer continuous with L1 of the spore wall constitutes the hyphal wall. L2 of the spore wall stops abruptly in the region of attachment and thus is not part of the more distant hyphal wall structure. The hyphal wall is thin (< 1 µm), so that it often breaks off or folds over the spore and thus may be hard to find. On a slide with 56 spores, only two showed the detail described above for the subtending hypha.
OCCLUSION: Very hard to see in many spores because the subtending hypha is so fragile; consists of a thickening of L2 of the spore wall to close off the spore contents. This layer does not appear to extend far (if at all) into the subtending hypha.
Mycorrhizae
Mycorrhizae in corn roots generally stain faintly in trypan blue, although intensity of the reaction varies from one region of a root to another (or among roots in a sample). The photos below are biased toward the most darkly staining regions for clarity in showing structures, so they are not representative of the full range of variation. Finely branched arbuscules and hyphae are darkest (and most noticeable) near entry points. Vesicles tend to stain darker than arbuscules or intraradical hyphae when present.
| Arbuscules in corn | |||
|---|---|---|---|
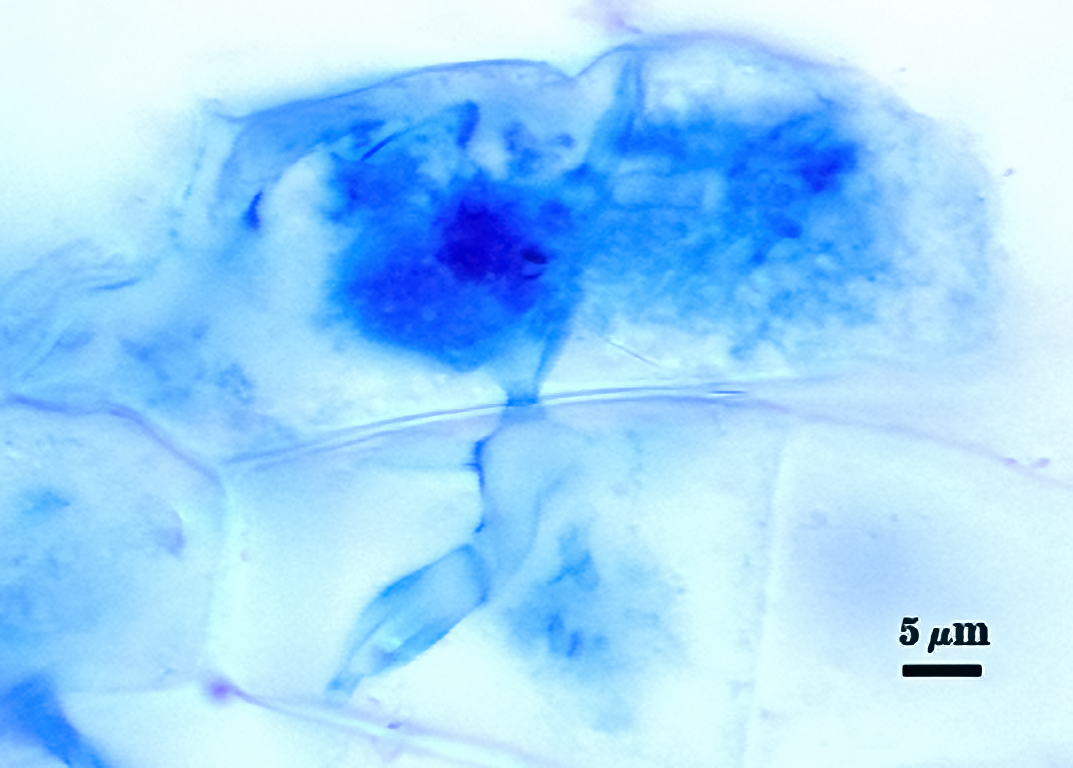 | 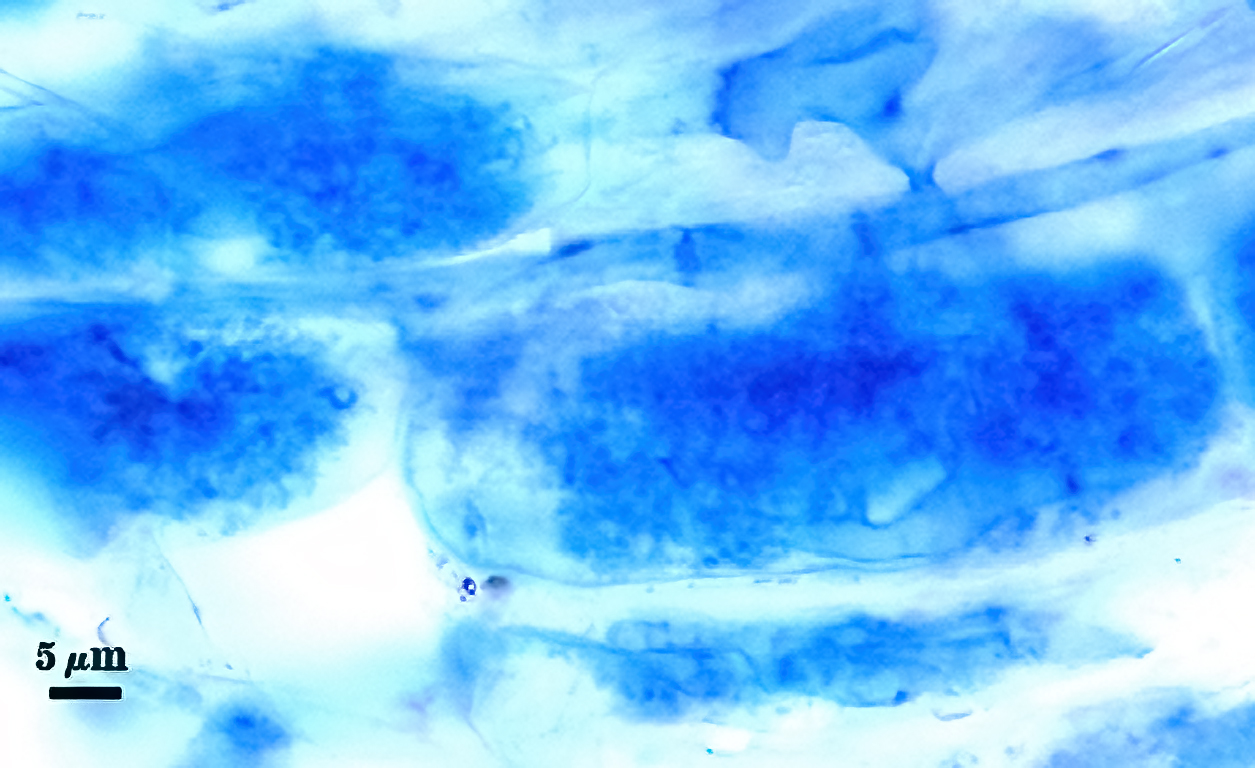 | 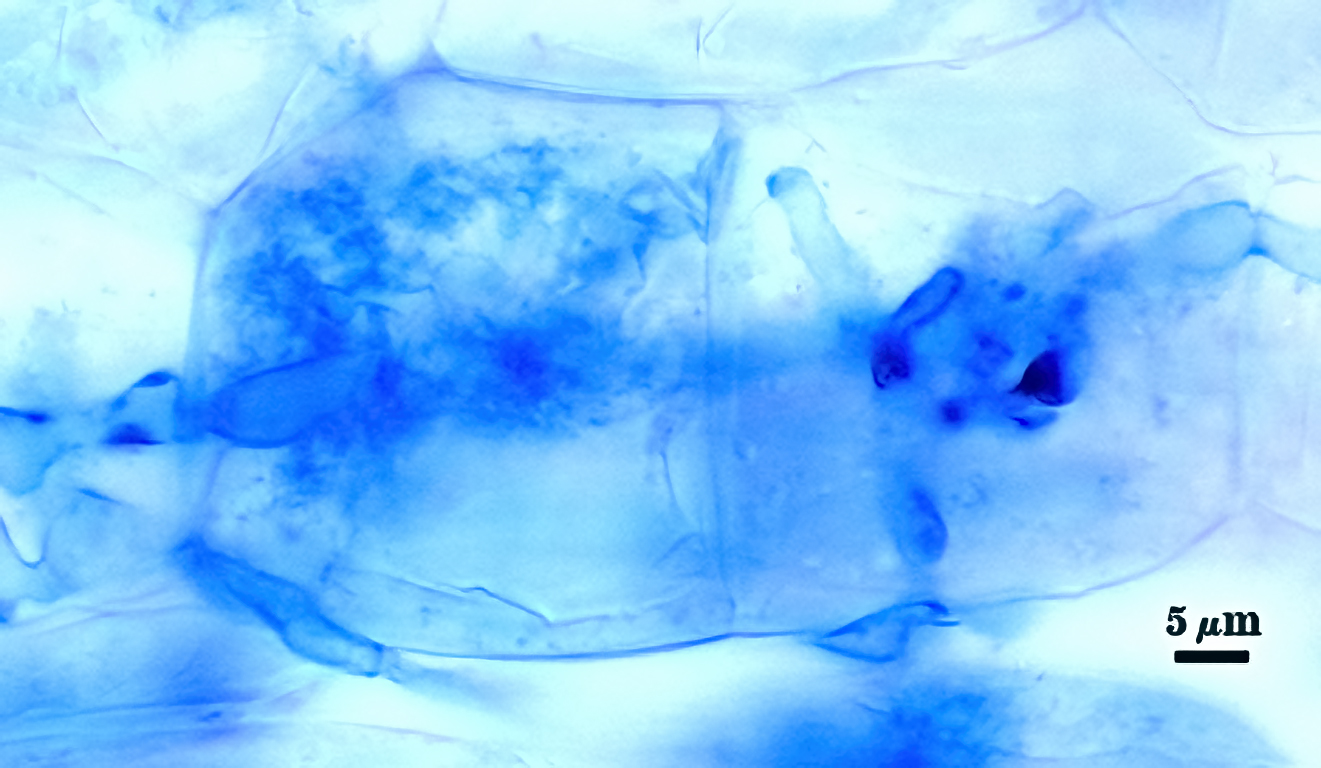 | 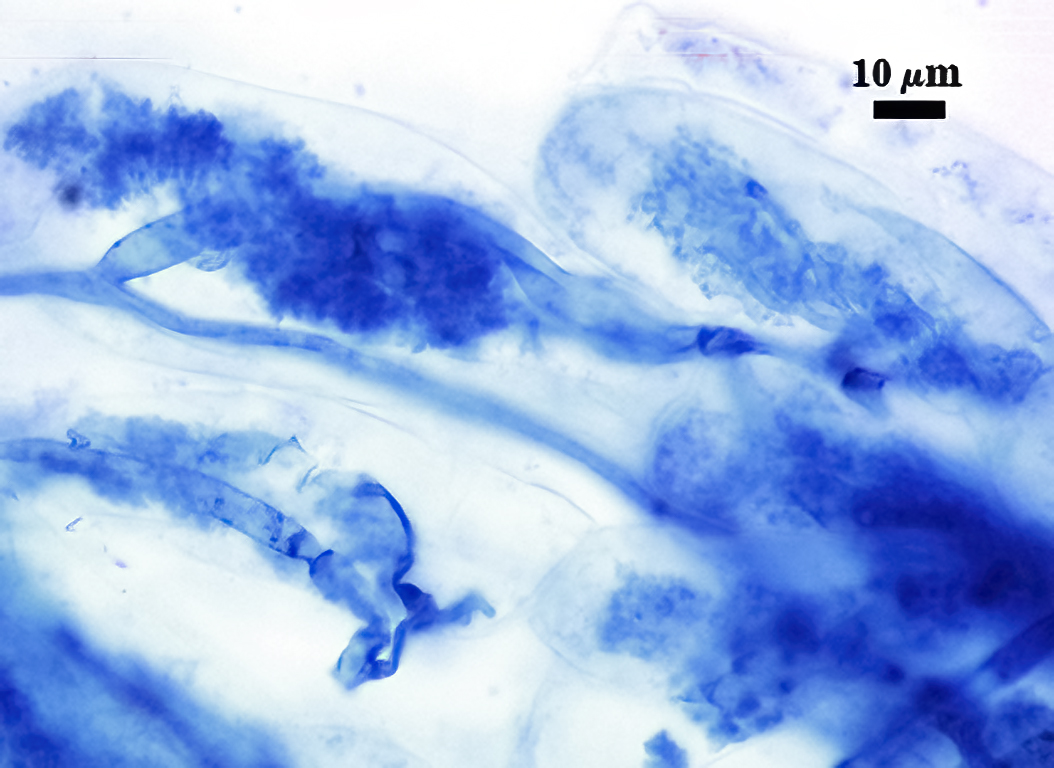 |
| Mycorrhizal structures in corn | |
|---|---|
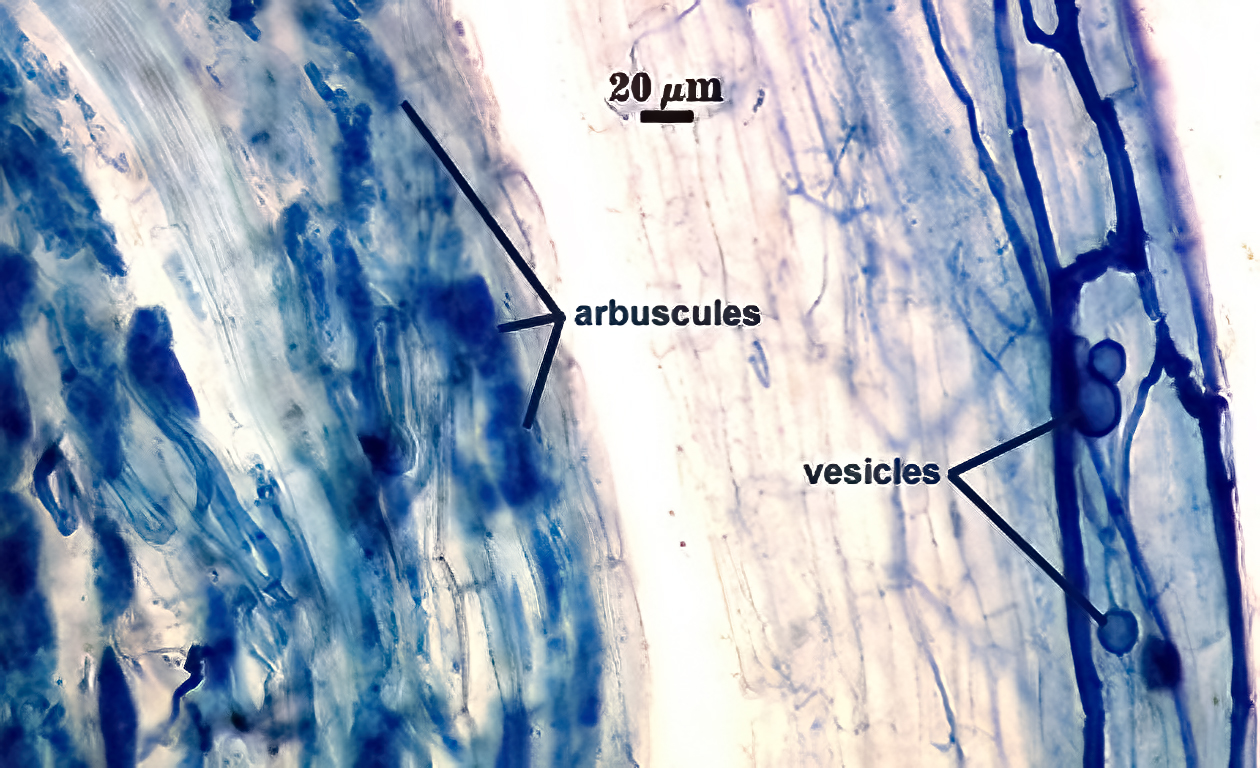 | 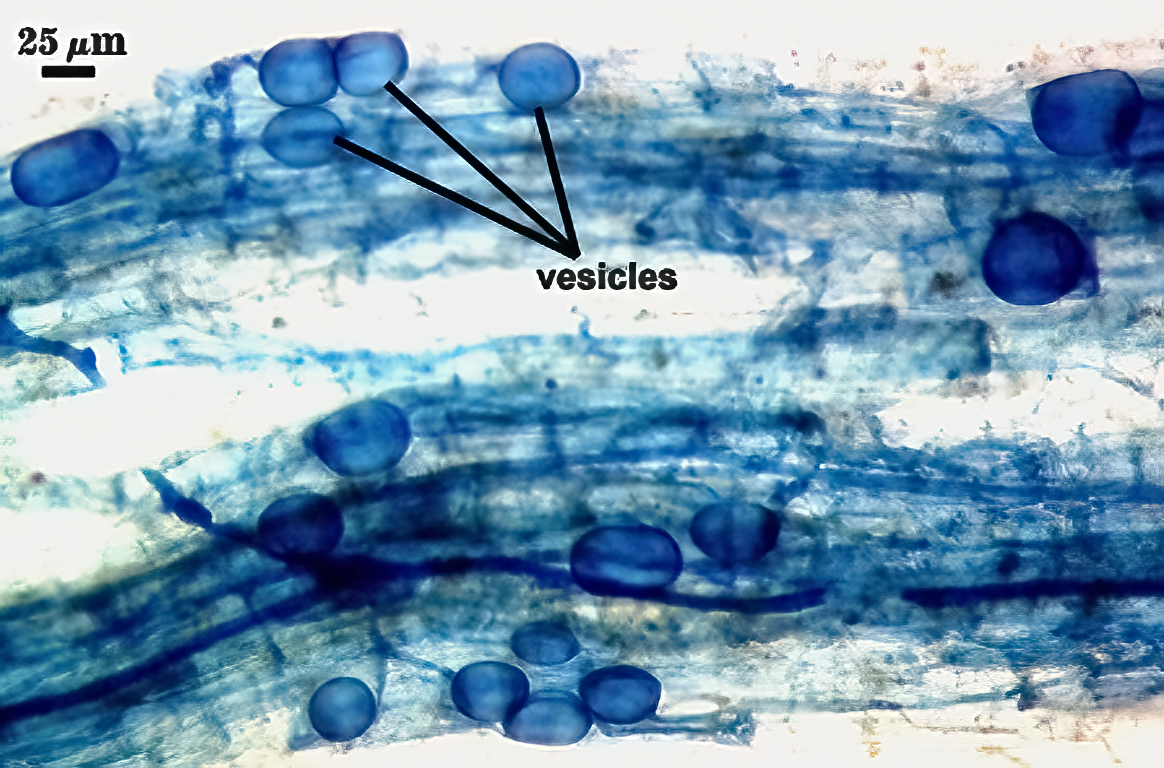 |
Notes
We have had this species in pot culture for over ten years, with isolates from quite distant locations (U.S., Africa, Hawaii). Walker and Pfeiffer (1996) described this species, with some features that we interpret differently: (i) the spore wall consisting of only two layers, with the laminate layer (L2) being somewhat flexible so that when crushed, it forms numerous tiny folds that give the impression of another “membranous” layer, so that no inner flexible layer is present and (ii) a wider range of staining variation than originally described as well as the presence of sporadic intraradical vesicles. These features were re-described by Kennedy et al. (1999) in order to clearly distinguish D. spurcafrom another species of similar morphology, D. eburnea. While these two species are similar, they can be readily distinguished with a practiced eye. Arizona workers (Kennedy, Stutz, and colleagues) as well as workers at INVAM have been able to easily separate the two species in monospecific cultures using two criteria together: (i) globose spores for D. spurca and tear-drop shaped spores for D. eburnea and (ii) spores with transparent centers for D. spurca (rendering them translucent in reflected light) and spores with dense opaque contents for D. eburnea (rendering them bright white in reflected light).
At the subcellular level, the two species differ in the following ways:
| Subcellular level | D. spurca | G. eburnea |
|---|---|---|
| L1 of spore wall | Separable from L2 | Adherent to L2 of spore wall |
| L2 of spore wall | Semi-flexible, with folds and some separation of sublayers | Slightly flexible, few folds, adherent sublayers |
| Subtending hypha | A continuation only of L1 of the spore wall | A continuation mostly of L1 of the spore wall |
| Occlusion structure | L2 of the spore wall at the spore base | L2 of the spore wall forms a re-curved septum a variable distance from the spore base |
We also have been somewhat unsure of how to interpret consistent differences in size range of spores among isolates into two distinct groups: those with spores rarely exceeding 80 µm in diam. and those with spores mostly greater than 80 µm diam (like the reference isolate). These groups are most strikingly illustrated in a side-by-side comparison of photos. The type species fits in the latter group, but we actually have more isolates of the former in monospecific pot cultures. LSU sequence data (Morton unpublished) groups both discrete (and consistently stable) size classes into one clade (species).
In our experience, D. spurca initially is fairly aggressive when cultured alone or together with other species, but tends to become attenuated after several successive culture generations. When present as part of a species mixture (e.g., trap cultures), it tends to sporulate profusely (like P. occultum) initially, but then sporulates less with successive generations and may eventually stop sporulation altogether (unlike P. occultum).
The images below can be uploaded into your browser by clicking on the thumbnail or can be downloaded to your computer by clicking on the link below each image. Please do not use these images for other than personal use without expressed permission from INVAM.
High Resolution Images | |
|---|---|
 |  |
 |  |
 |  |
 |  |
Reference
- Pfeiffer, C. M., C. Walker, and H. E. Bloss. 1996. Glomus spurcum: A new endomycorrhizal fungus from Arizona. Mycotaxon 59:373-382
- Kennedy, L. J., J. C. Stutz, and J. B. Morton. 1999. Glomus eburneum and G. luteum, two new species of arbuscular mycorrhizal fungi, with emendation of G. spurcum. Mycologia 91:1083-1093.