Claroideoglomus claroideum
(reference accession SC186)
= Glomus fistulosum
= Glomus maculosum
= Glomus multisubstensum
Whole Spores
 COLOR: Cream (0-0-10-0) to light yellow (0-0-40-0)
COLOR: Cream (0-0-10-0) to light yellow (0-0-40-0)
SHAPE: Globose to subglobose
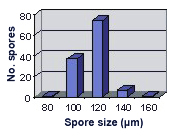
SIZE DISTRIBUTION: 80-160 µm, mean = 115 µm (n = 120)
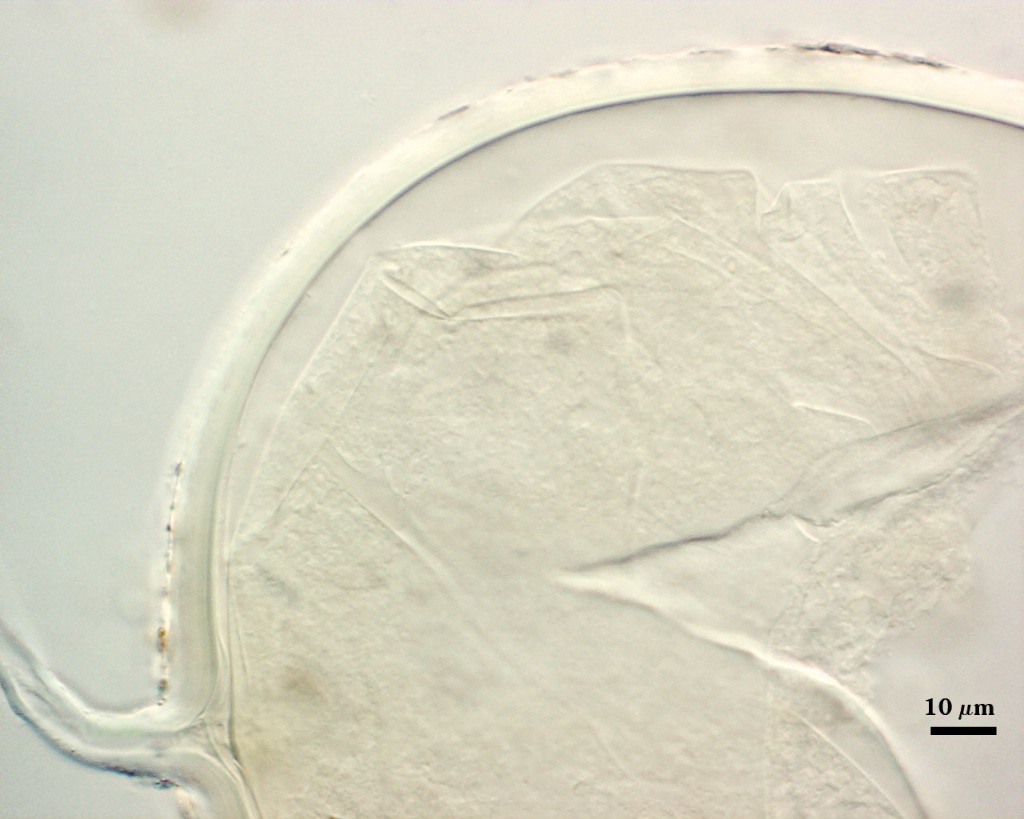
Subcellular Structure of Spores
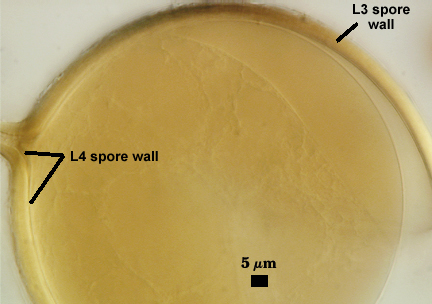
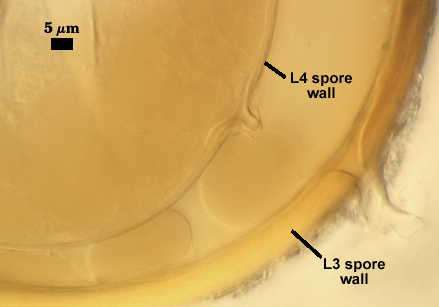
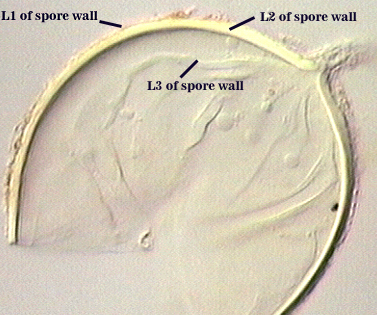
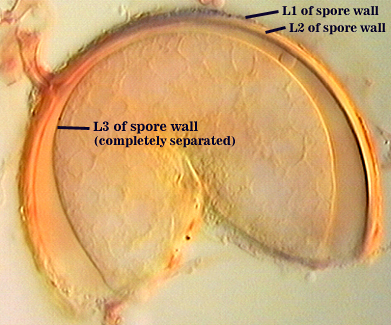
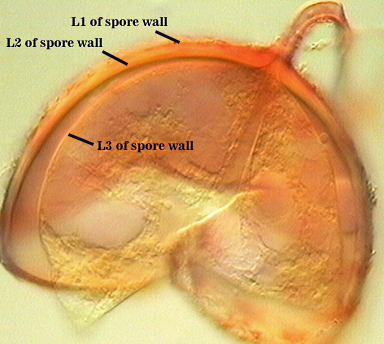
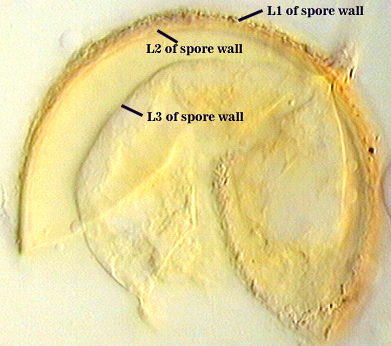
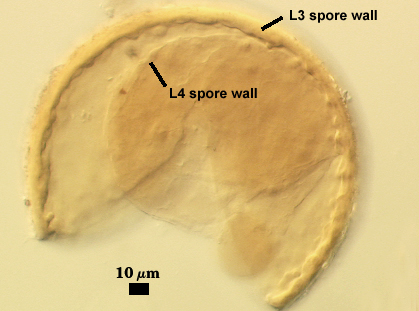
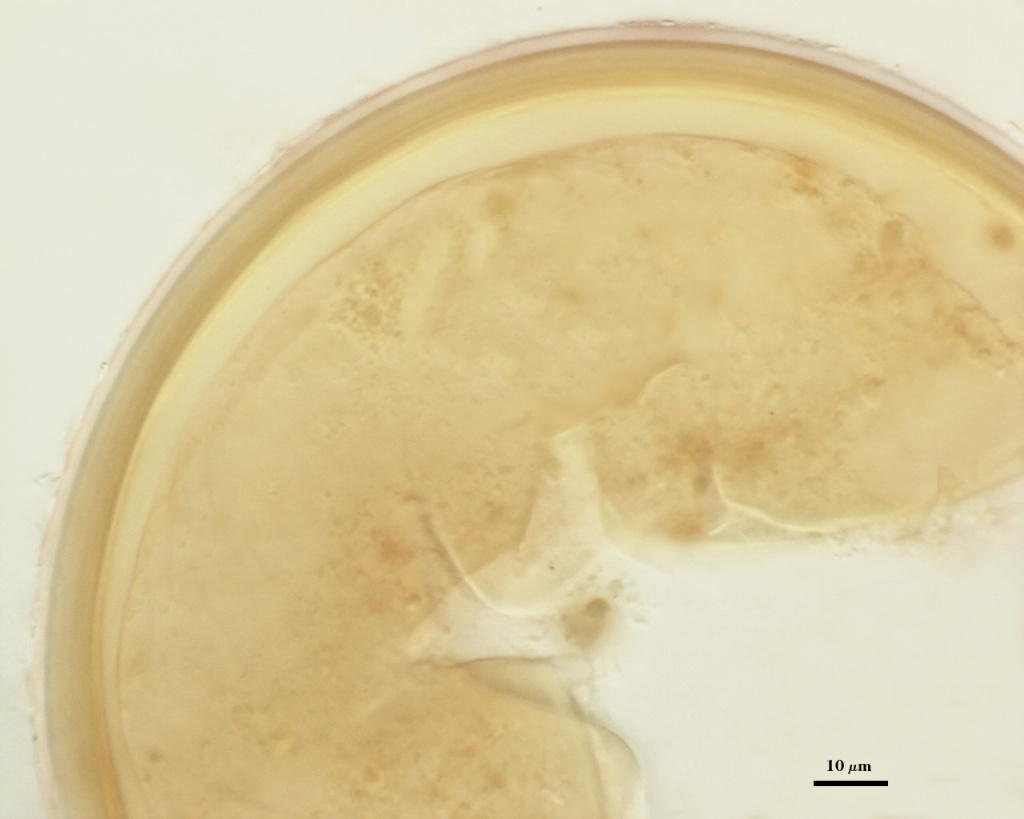
SPORE WALL: Four layers (L1, L2, L3 and L4), only the first two present in the most juvenile spores (left photo below) and continuous with the juvenile wall of the subtending hypha. L3 develops next in both the subtending hypha and spore, gradually thickening (right photo below) to eventually make up most of the spore wall thickness. L4 is last to form, originating as part of the subtending hyphal wall, but only in the region of the spore base.
| Smashed Spheres | |
|---|---|
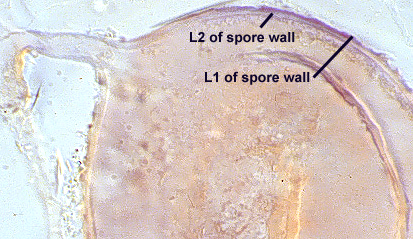 |  |
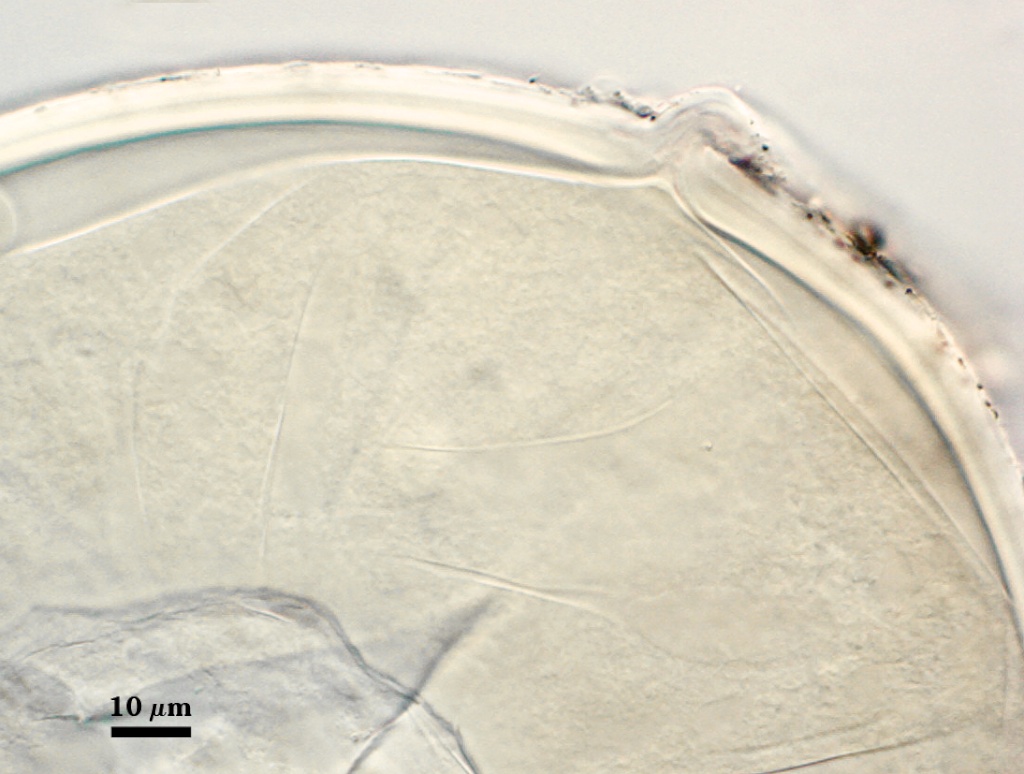
L1: Hyaline mucilagenous layer, 0.6-1.8 µm thick in young spores; producing a pink (0-30-20-0) to darker pink (0-40-20-0) reaction in Melzer’s reagent. It often is difficult to distinguish from L2 because both layers are so tightly adherent. This layer appears granular as it decomposes, usually sloughing completely in mature spores (along with L2).
L2: Hyaline, formed in juvenile spores with L1, 0.6-2.0 µm thick, degrading and sloughing concomitant with L1. As this layer degrades, it appears to attract organic debris which can accumulate on the spore surface; no reaction in Melzer’s reagent.
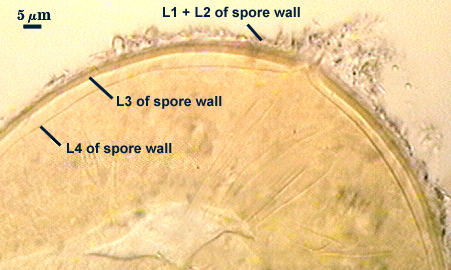
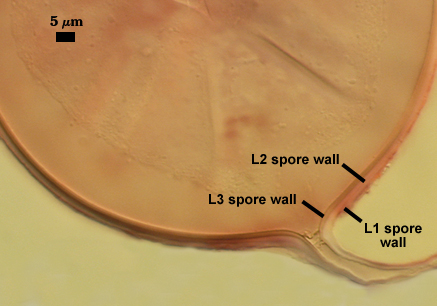
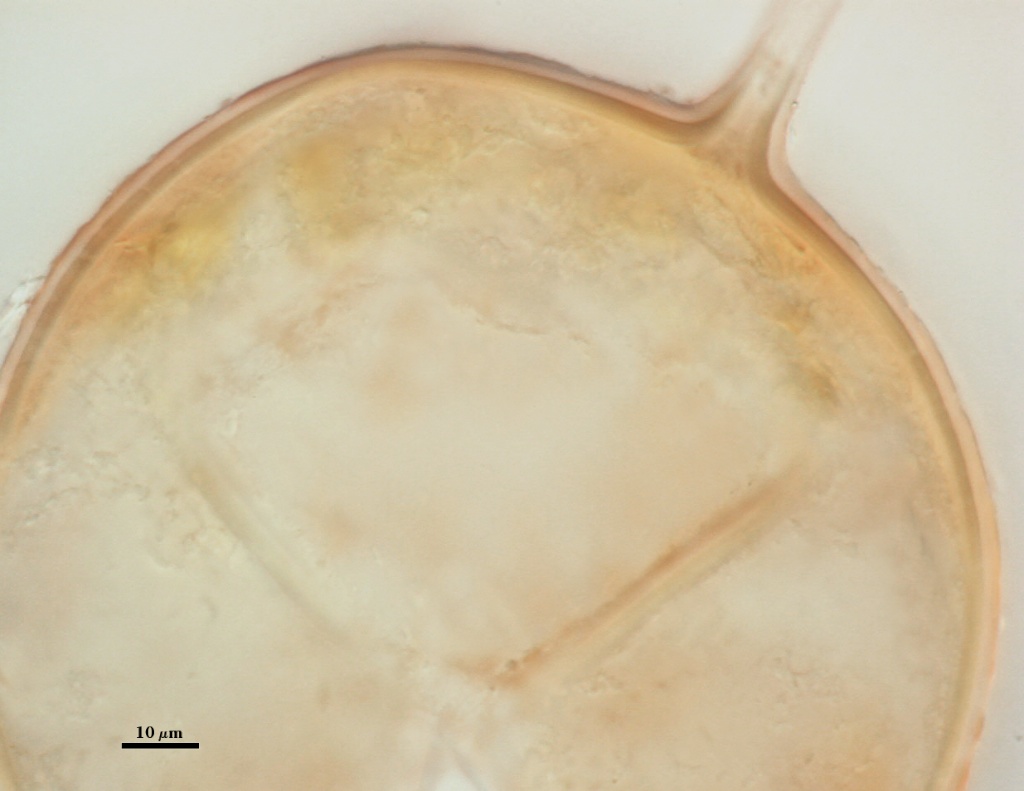
L3: A layer consisting of thin and tightly adherent pale yellow (0-0-20-0) sublayers (or laminae), 2.8-6.2 µm thick (mean of 3.8 µm) in mature spores.
| L4 spore wall | ||
|---|---|---|
|
|
|
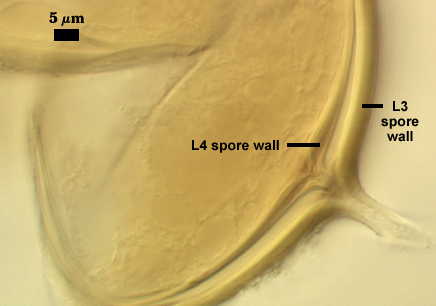
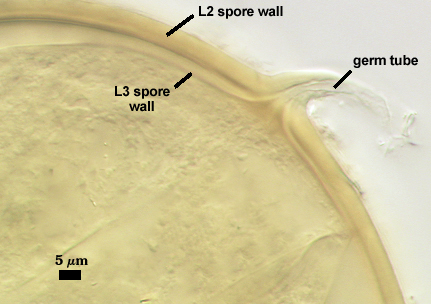
L4: A layer concolorous with the laminate layer that can often appear quite thin (< 0.5 µm) or thicken with sublayers to almost 3 µm; producing folds when it is very thin (see gradation from thin to thick in photos at right). Its appearance closely resembles that of a flexible inner germinal wall (= “membranous” wall), but it extends into the subtending hypha as part of the hyphal wall. It is not even analagous to a germinal wall even though similar in appearance because it has no role in germination processes. Since it originates as part of the subtending hypha, it is considered as the innermost layer of the spore wall. It is not considered as a sublayer of L3 because it consistently separates to varying degrees from the spore wall. In some spores it breaks away completely from the spore wall, and it has a small protrusion where it was attached to the subtending hyphal wall (see middle photo above).
| Spores mounted in PVLG |
|---|
| Spores mounted in PVLG and Melzer’s reagent (1:1 v/v) | |||
|---|---|---|---|
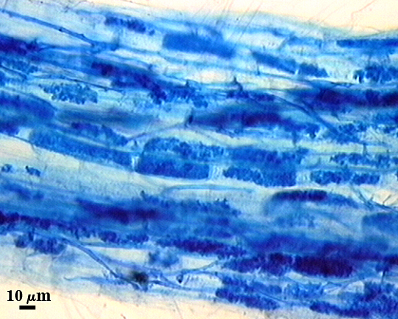
Subtending Hypha
SHAPE: Cylindrical to slightly flared (see photos above).
WIDTH: 6-8 µm (mean = 6.9 µm).
WALL STRUCTURE: Three layers (L1, L2 and L3) continuous with the outer three layers of the spore wall, 1.2-3.2 µm thick in the region of attachment, tapering within 15 µm of the spore to less than 1.0 µm thick. L1 and L2 usually absent from the surface of mature spores. In many spores, the hyphal wall is so fragile that it breaks from the spore and cannot be seen. L4 does not extend into the hypha beyond the “septum” that partitions spore from hyphal cytoplasm.
OCCLUSION: Either a sublayer of the laminate layer (L3) and L4 or L4 alone bridge the hyphal pore and form what resembles a recurved “septum”.
Germination
A germ tube emerges from the lumen of the subtending hypha. Walker and Vestberg (1998) report germination also through the spore wall, but we have not observed this in any of the accessions in the collection.
Mycorrhizae
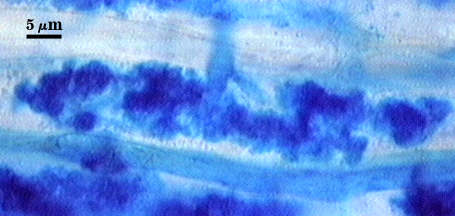
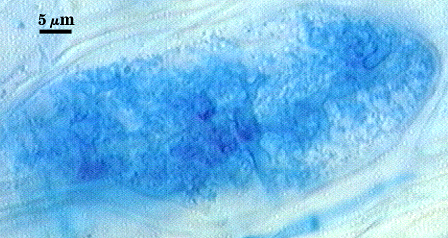
Source host for this analysis was corn (Zea mays L.). Arbuscules branch from intraradical hyphae 2.5-4 µm in diameter, staining darkly in trypan blue when fully formed and functional (left photo), becoming more diffuse and lightly stained as they begin to undergo senescence and dissolution (right photo). Branch hyphae from the main trunk of the arbuscule narrow incrementally to numerous fine tips.
Intraradical hyphae of a mycorrhiza also stain darkly in trypan blue. They tend to grow intercellularly between cortical cells, with the hyphal network consisting of numerous “H and h” branches and occasional coils. Some hyphae will have the appearance of projections, but they often are remnants of the trunk hyphae of intracellular arbuscules. Vesicle development is sporadic and patchy, rarely abundant in any particular infection unit. Many mycorrhizae consist of few to no vesicles. Occurrence and abundance of vesicles may be as much an expression of environmental as genetic determinants.
| Arbuscules in corn | |
|---|---|
| Mycorrhizae in corn | |
|---|---|
Notes
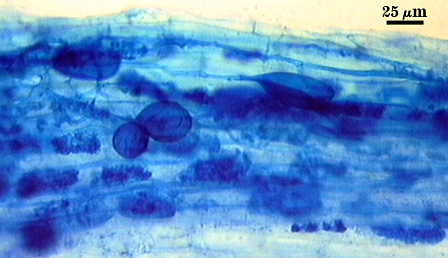
Spores in the type specimen of G. claroideum are partially decomposed and darkly pigmented from long-term storage in lactophenol. Spores broken and mounted in PVLG do not show any evidence of the sloughing (L1) layer, and only a few show an adherent L3 layer of the spore wall.
The complex spore wall structure of Glomus fistulosum, described by Skou and Jakobsen (1989), appears to be an artifact of the condition of spores and the way they were crushed when mounted on glass slides. Walker and Vestberg (1998), recognizing this, formally synonymized these two species.
Old spores, when parastized, also form thickenings on L4 (see photo at right) so that they take on the properties described for the spore wall of Glomus maculosum (Miller and Walker, 1989). The “maculosa” of G. maculosum spores have long been suspected of being an artifact of parasitism, a view also shared by Walker and Vestberg (1998) and they synonymized G. maculosum with G. claroideum.
Walker and Vestberg (1998) also synonymized Glomus multisubstensum (Mukerji et al. 1983) with G. claroideum. We support this move in the absence of any direct experience with the species.
The images below can be uploaded into your browser by clicking on the thumbnail or can be downloaded to your computer by clicking on the link below each image. Please do not use these images for other than personal use without expressed permission from INVAM.
High Resolution Images | |
|---|---|
 |  |
 |  |
 |  |
Reference
- Miller, D. D. and C. Walker. 1986. Glomus maculosum sp. nov. (Endogonaceae): An endomycorrhizal fungus. Mycotaxon 26:217-227.
- Mukerji, K.G., M. Bhattacharjee, and J. P. Tewari. 1983. New species of vesicular-arbuscular mycorrhizal fungi. Trans. Br. Mycol. Soc. 81:641-643.
- Skou, J. P. and I. Jakobsen. 1989. Two new Glomus species from arable land. Mycotaxon 36:273-282.
- Walker, C. and M. Vestberg. 1998. Synonymy amongst the arbuscular mycorrhizal fungi: Glomus claroideum, G. maculosum, G. multisubstensum and G. fistulosum. Annals of Botany 82:601-624.