Diversispora epigaea
(reference accession IT104)
Sporocarps
According to Daniels and Trappe (1979), in their description of G. epigaea, sporocarps are irregular, 2-8×3-15 mm in size, sometimes fused into larger masses, dull brownish yellow to brown in color; they arise from a basal pad of pale grayish yellow, loose mycelium with a few interspersed spores; no peridium is formed.
| SPOROCARPS | |
|---|---|
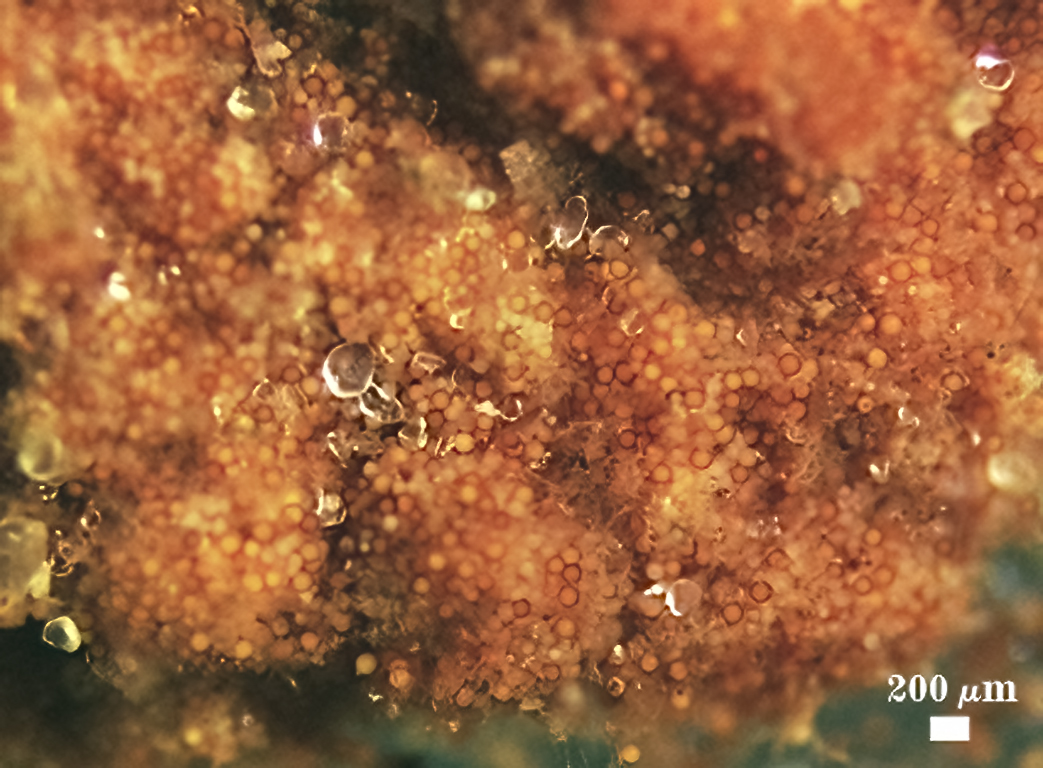 |  |
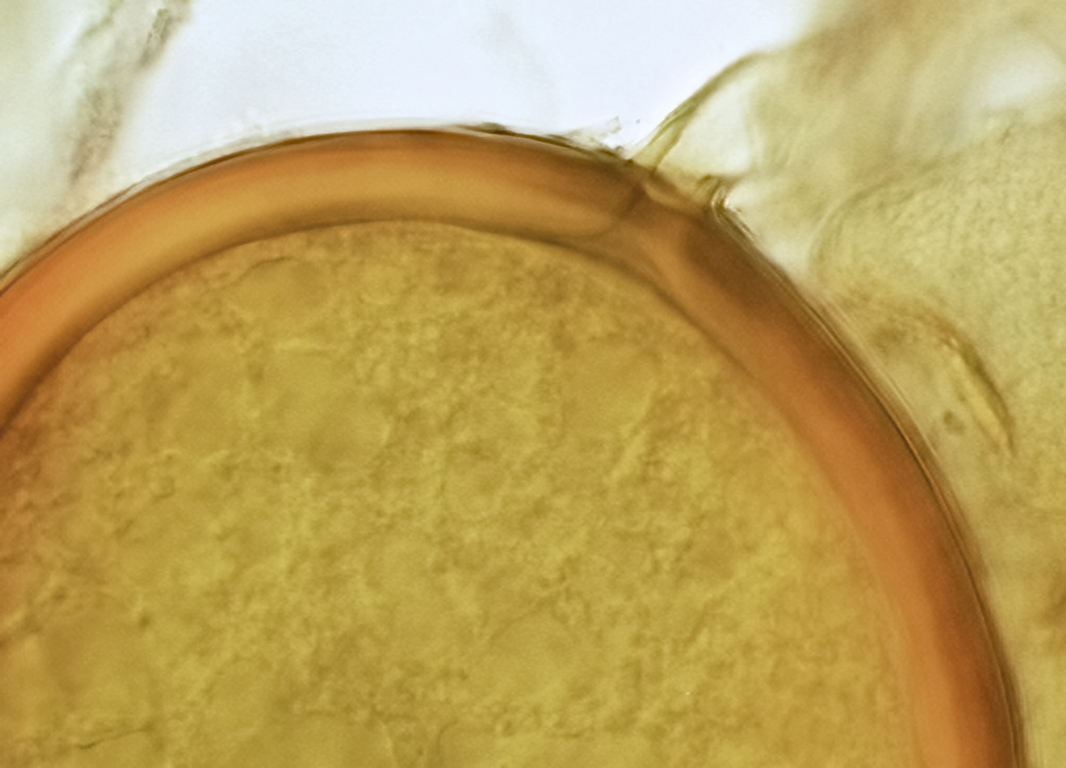
Whole Spores
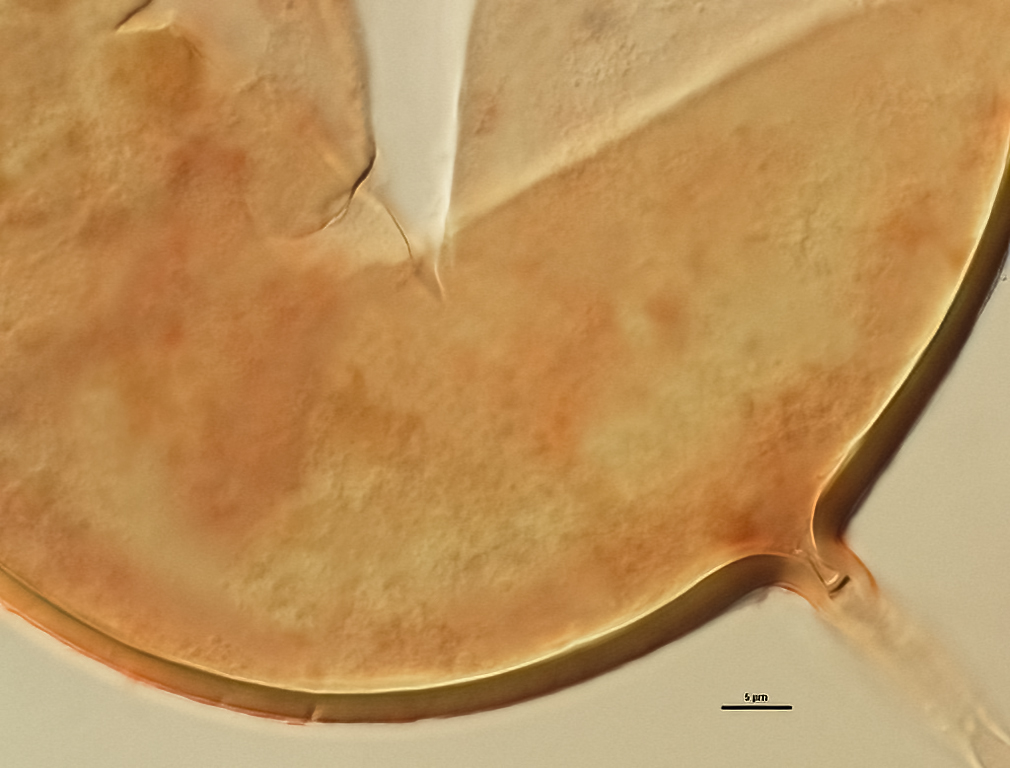
COLOR: Orange (0-10-90-0) to red brown(0-60-100-0) in sporocarps (mature). When produced singly in pot culture, spores are white, cream-colored (0-0-20-0) to pale orange-yellow (0-10-60-0).
SHAPE: Globose, subglobose, sometimes ovoid.
| WHOLE SPORES | |||
|---|---|---|---|
 |  | 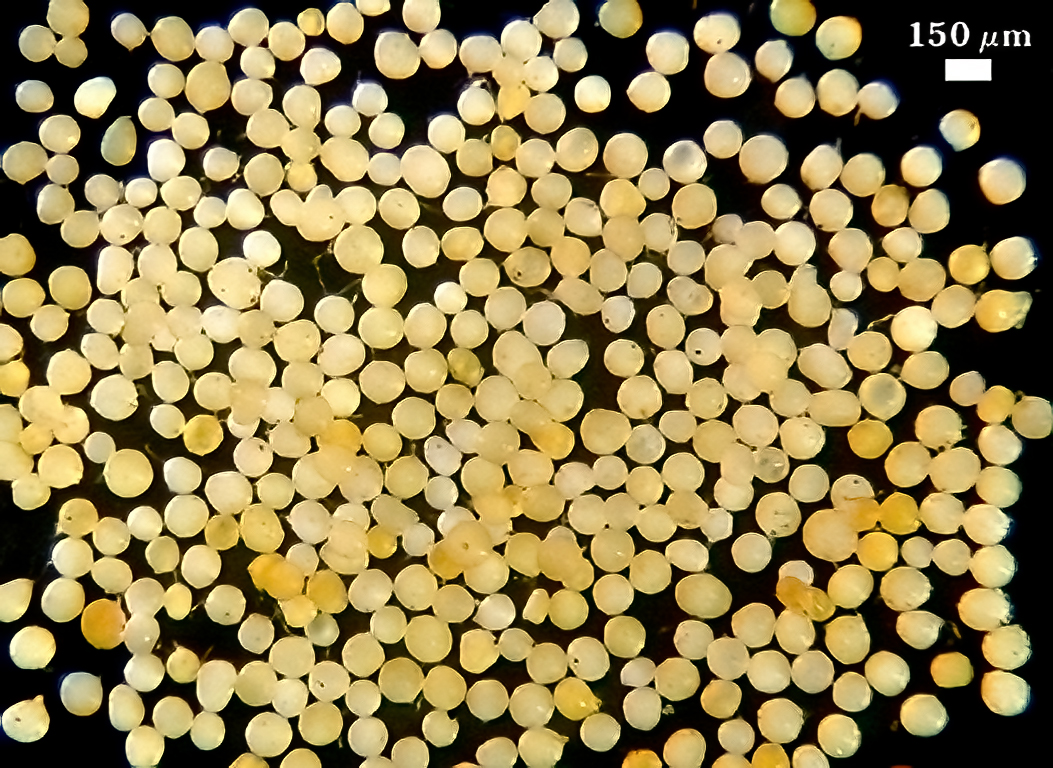 | |
SIZE DISTRIBUTION: 60-160 µm, mean = 129 µm (n = 120). Six-month-old pot cultures of two accessions (IT104, KS210) failed to produce sporocarps on sudangrass in a low organic matter (< 1%) sandy soil. Instead, numerous single spores form abundantly around roots amongst masses of extramatrical hyphae (see photos below). Repeated subcultures on different hosts (leek, corn) did not change this sporulation behavior.
| SIZE DISTRIBUTION | |
|---|---|
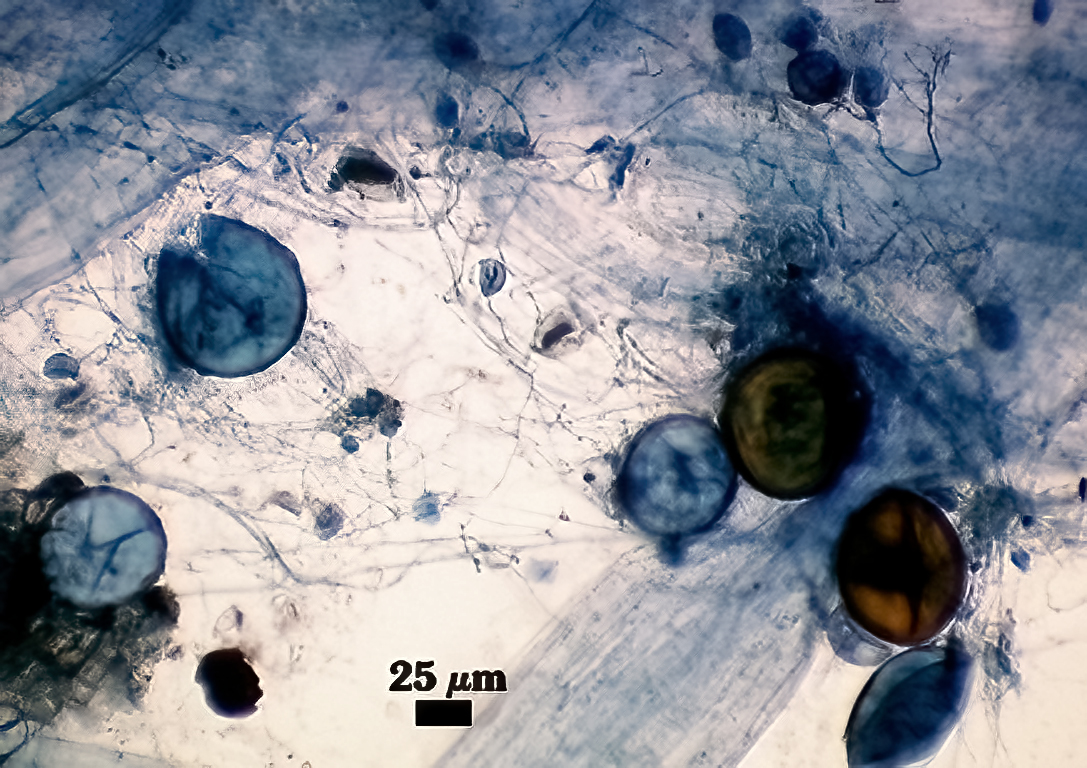 | 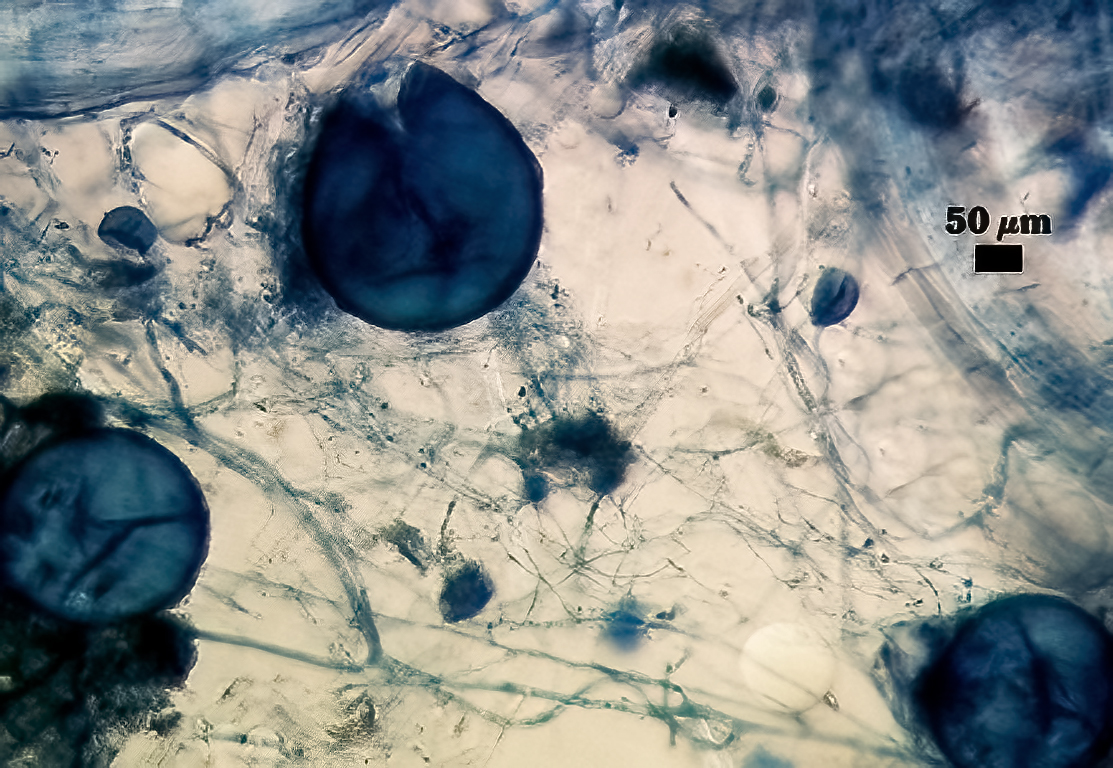 |
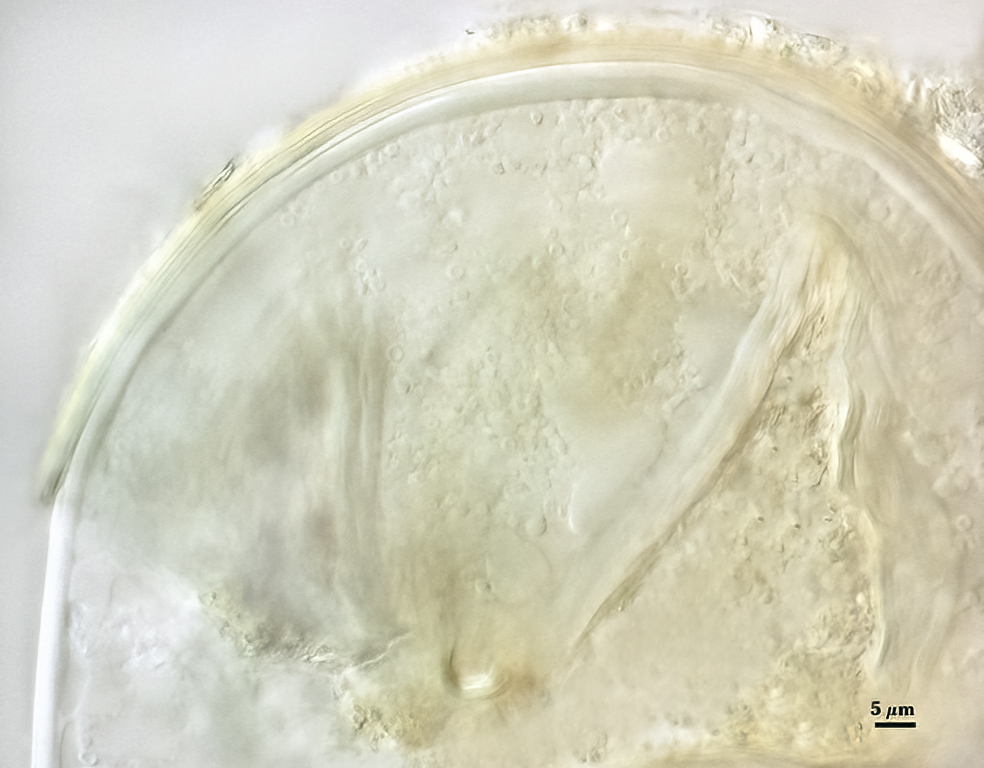
Subcellular Structure of Spores
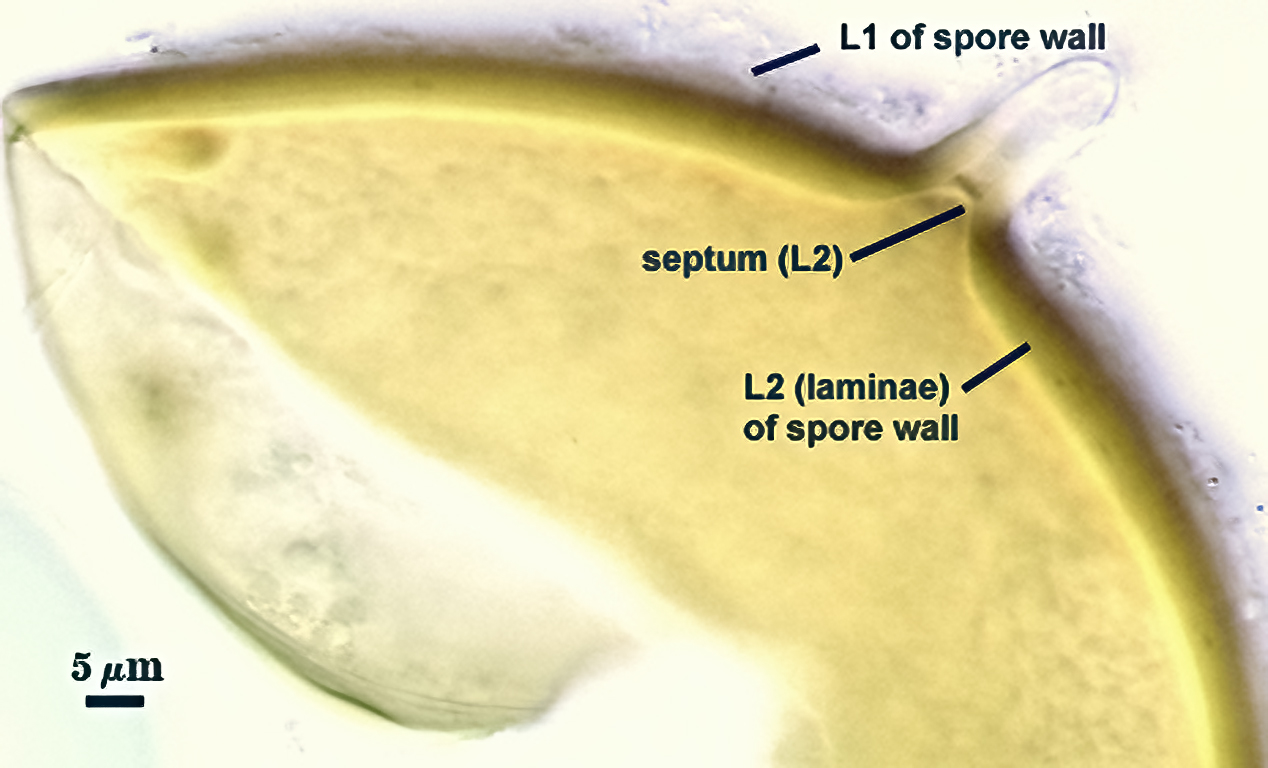
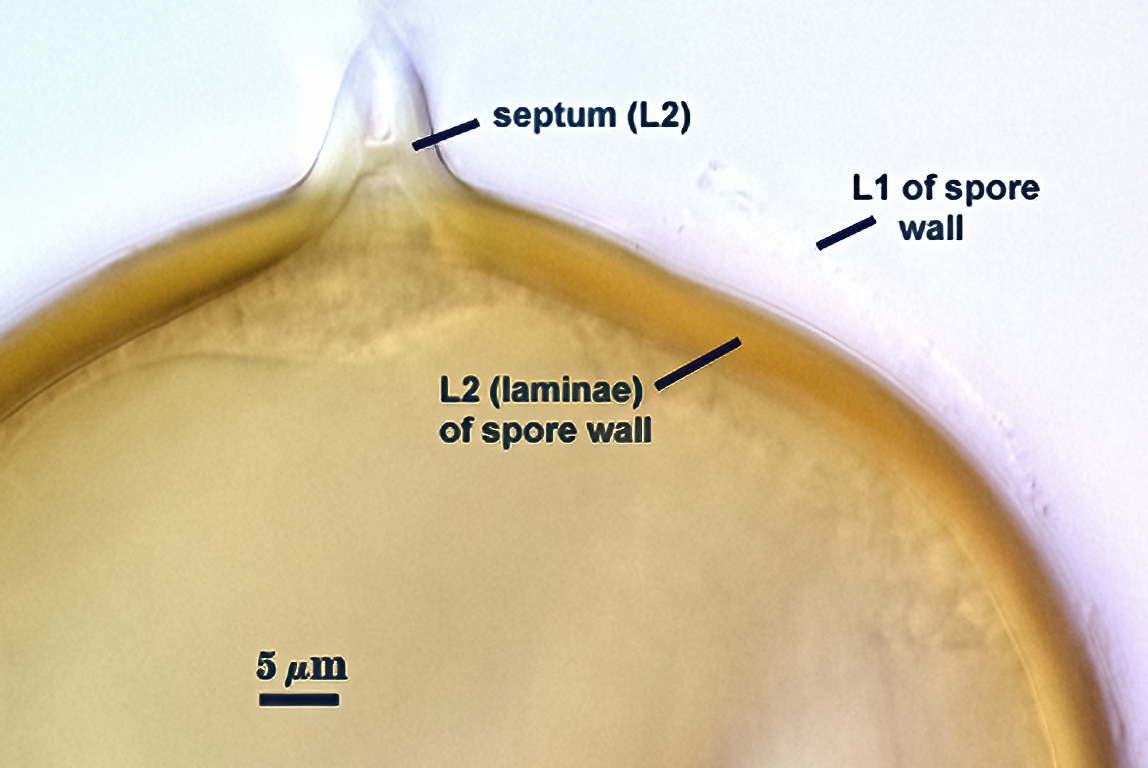
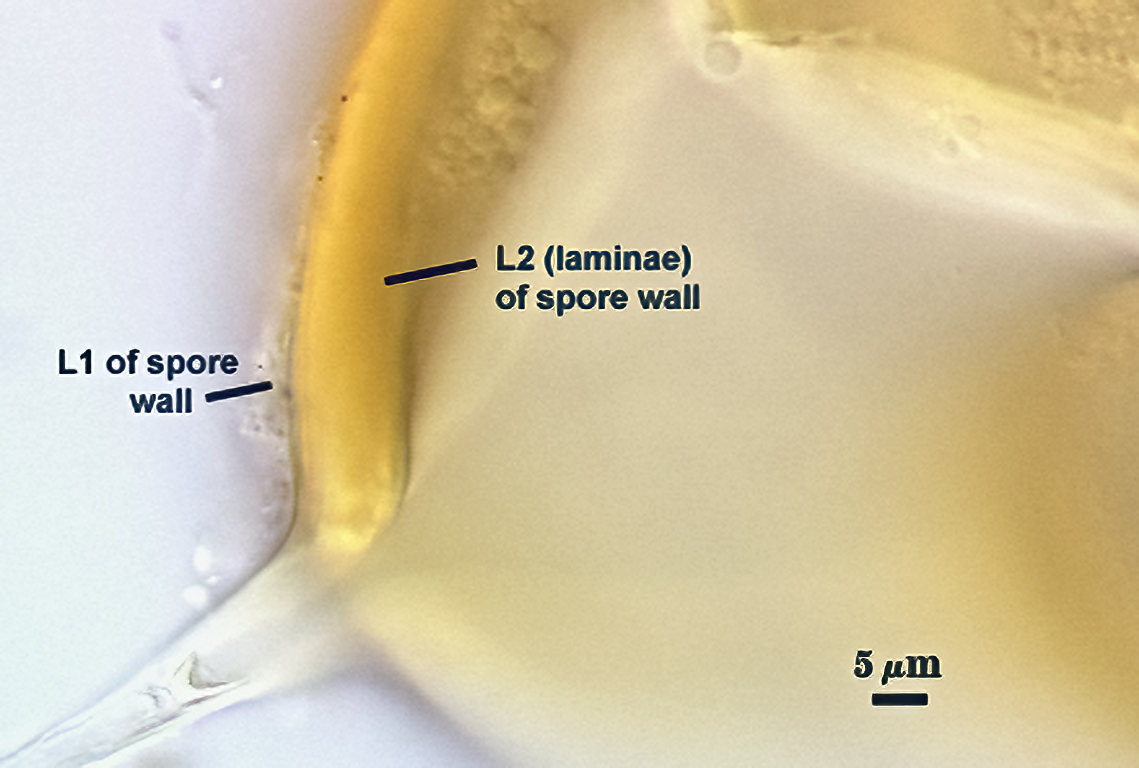
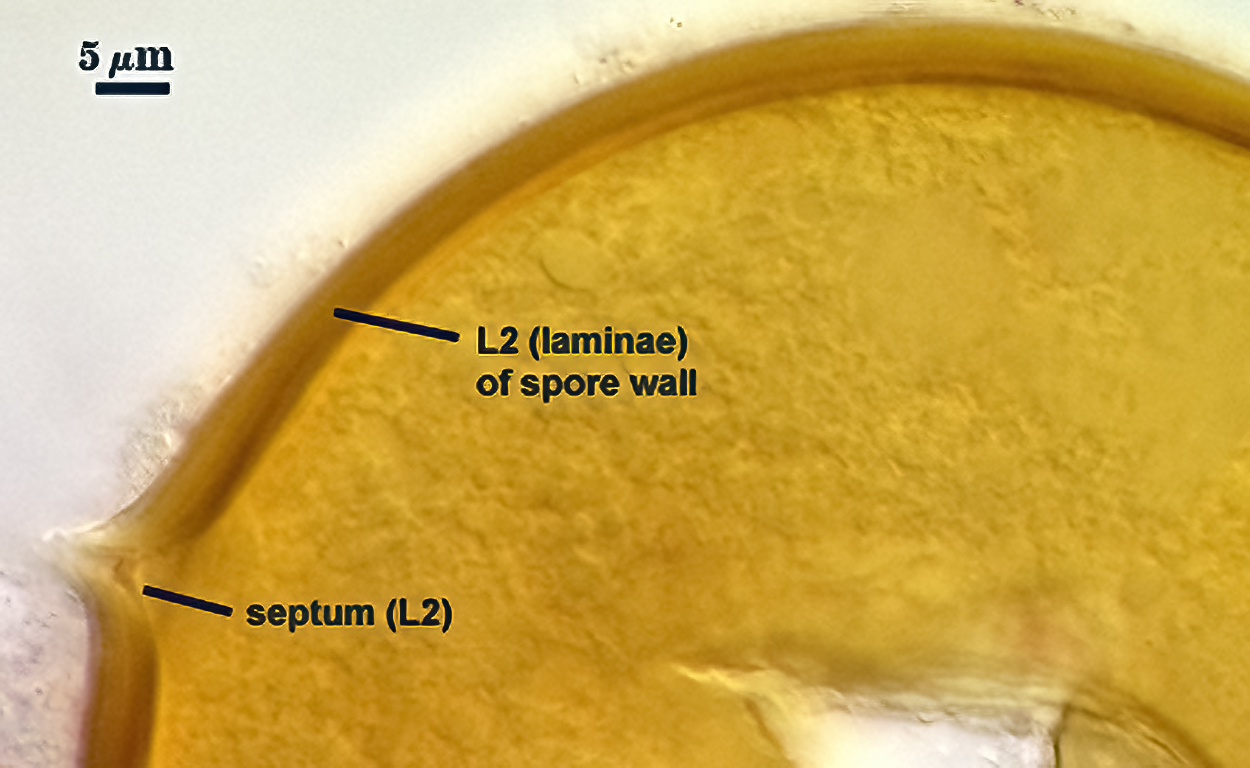
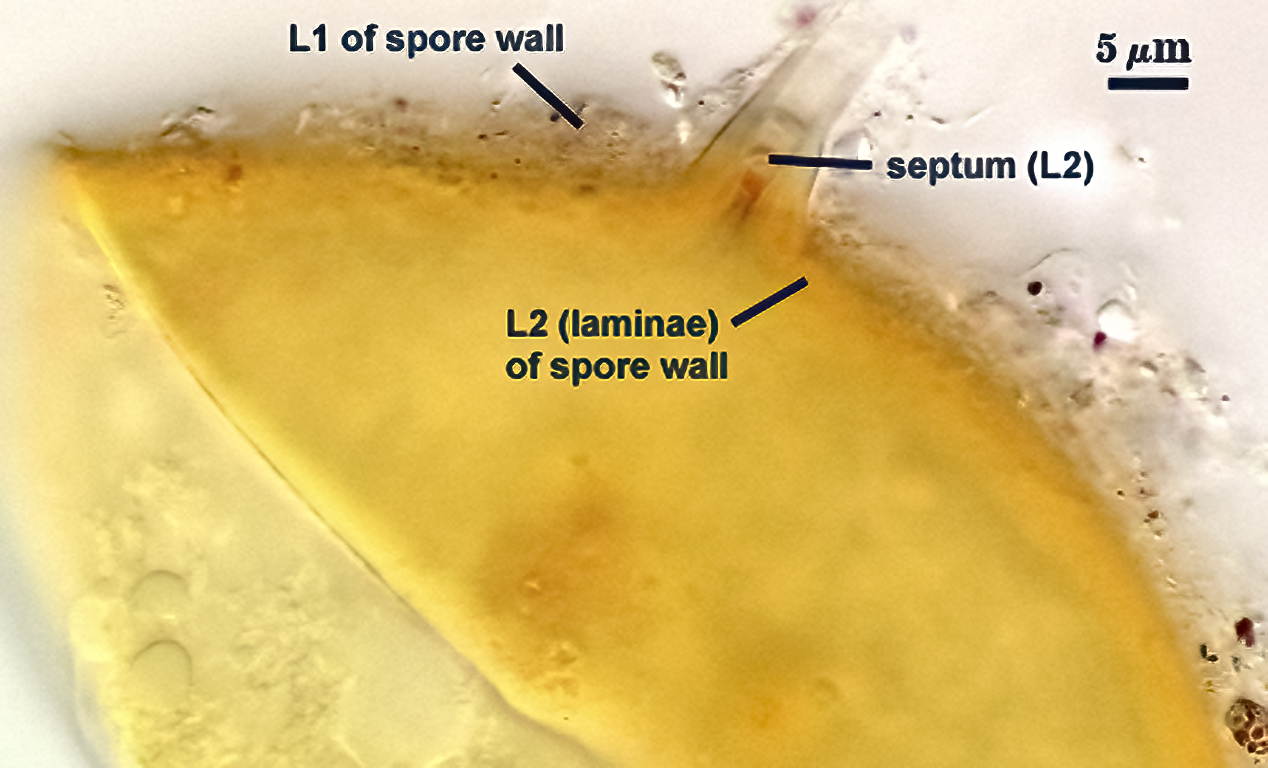
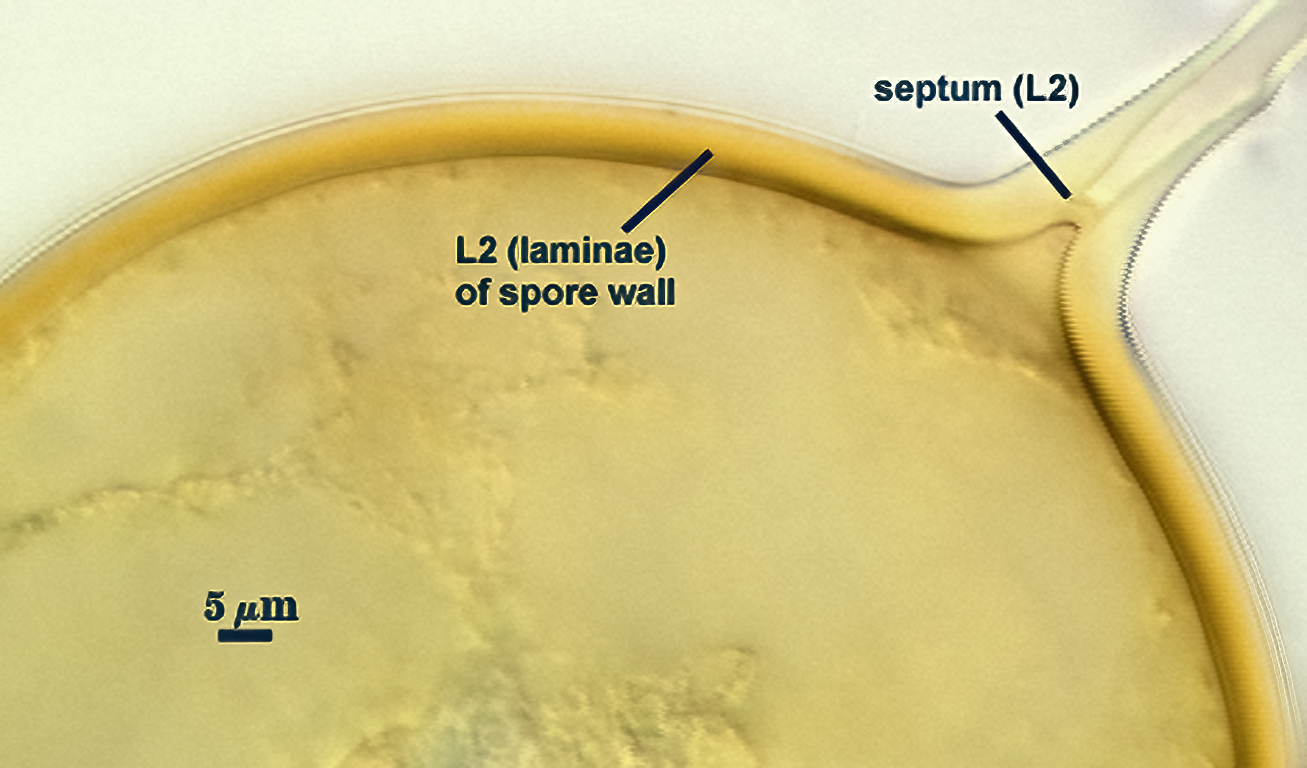
SPORE WALL: Consisting of two layers (L1 and L2) that differentiate consecutively as spores develop, composite thickness 5-10 µm.
L1: An outer layer adherent to the layer beneath it (L2), semi-permanent, subyhyaline, 0.5-1 µm thick; no reaction in Melzer’s reagent.
L2: A layer consisting of thin adherent sublayers (or laminae), pale yellow-brown (0-10-20-0) to orange-brown (0-40-100-0) in color; 4-9 µm thick.
| In PVLG | ||
|---|---|---|
 |  |  |
| In PVLG + Melzer’s reagent (1:1 v/v) | ||
|---|---|---|
 |  |  |
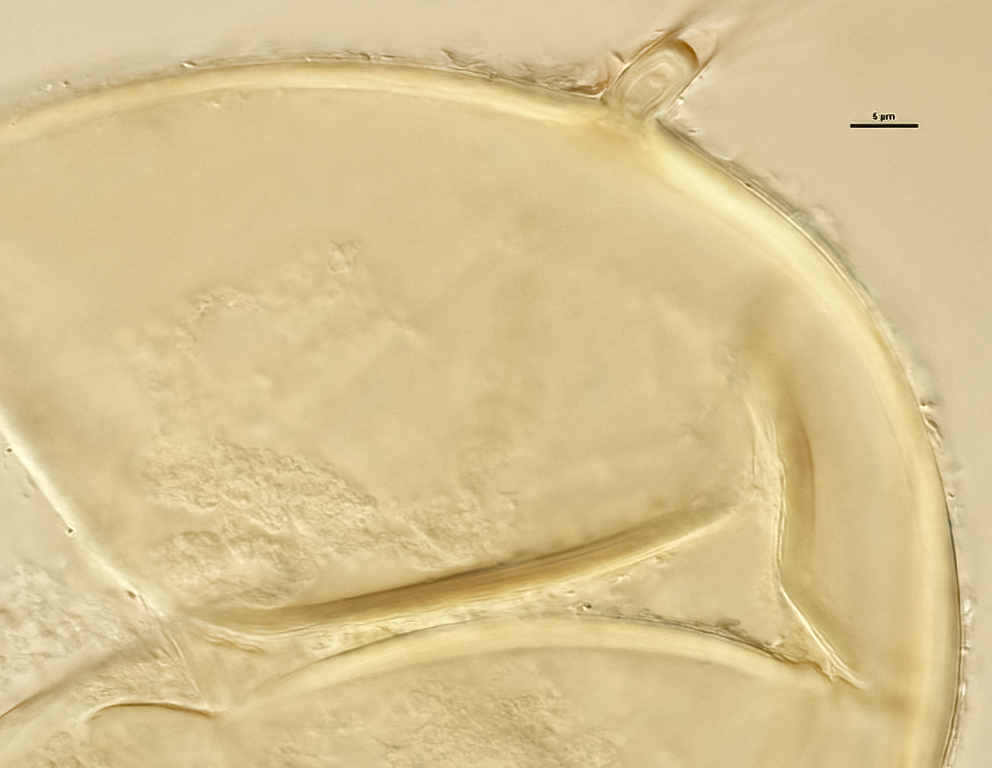
Subtending Hypha
SHAPE: Cylindrical to slightly flared (see photos above).
WIDTH: 4-7 µm (mean = 5.4 µm).
COMPOSITE WALL THICKNESS: 1-2.2 µm at spore base
WALL STRUCTURE: Two layers (L1 and L2) continuous with the two layers of the spore wall.
L1: The outer layer, subhyaline, usually present only near point of attachment of mature spores.
L2: Inner layer continuous with L2 of the spore wall, pale orange-brown (0-20-40-0).
OCCLUSION: Recurved septum positioned at the innermost sublayer of the laminate layer (L2), giving the appearance of being “inserted” (Daniels and Trappe, 1979).
Germination
A germ tube emerges from the lumen of the subtending hypha.
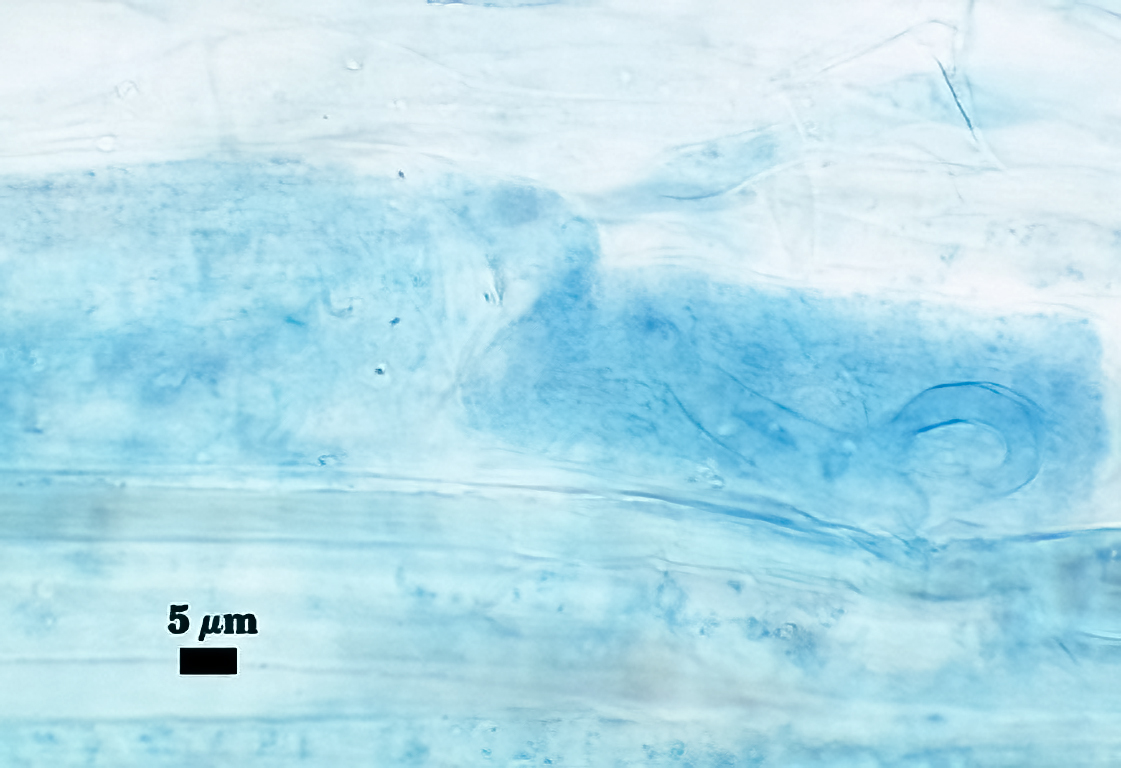
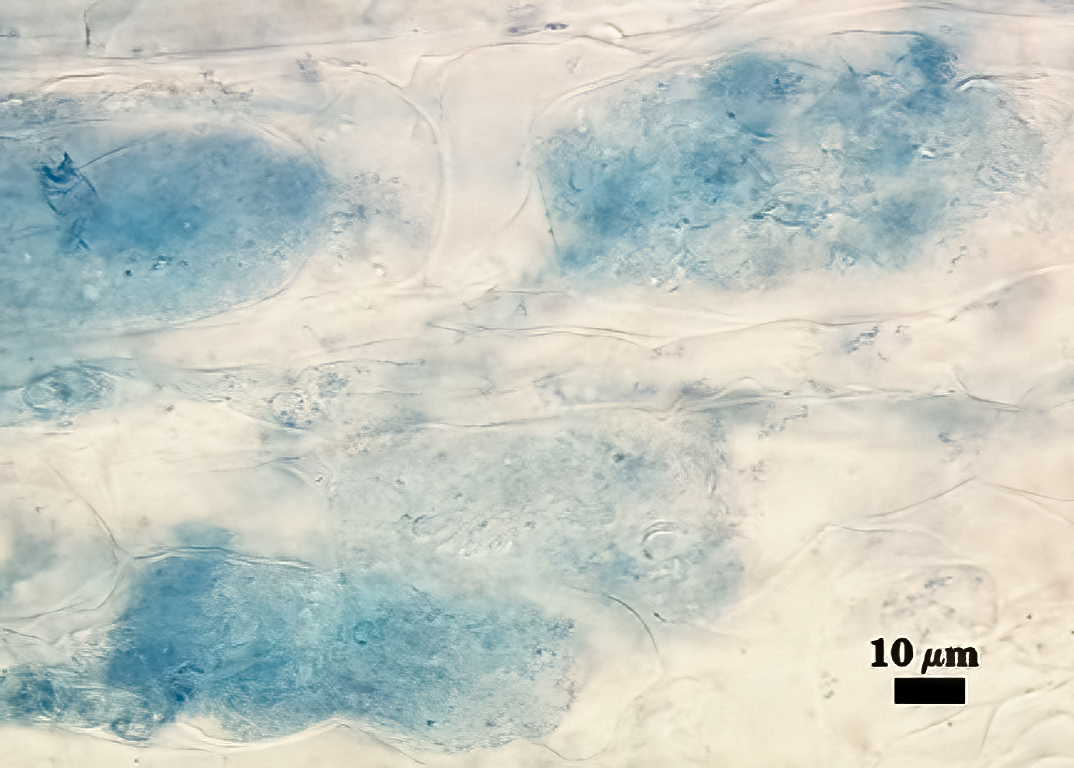
Mycorrhizae
All fungal structures (arbuscules, intraradical hyphae) stain only faintly in Direct Blue (or Acid Fuchsin), regardless of host species. No vesicles were observed in cultures grown at least 4 months. Coiled hyphae were common near entry points.
| Arbuscules in corn roots - Coiled hyphae | ||
|---|---|---|
 |  | 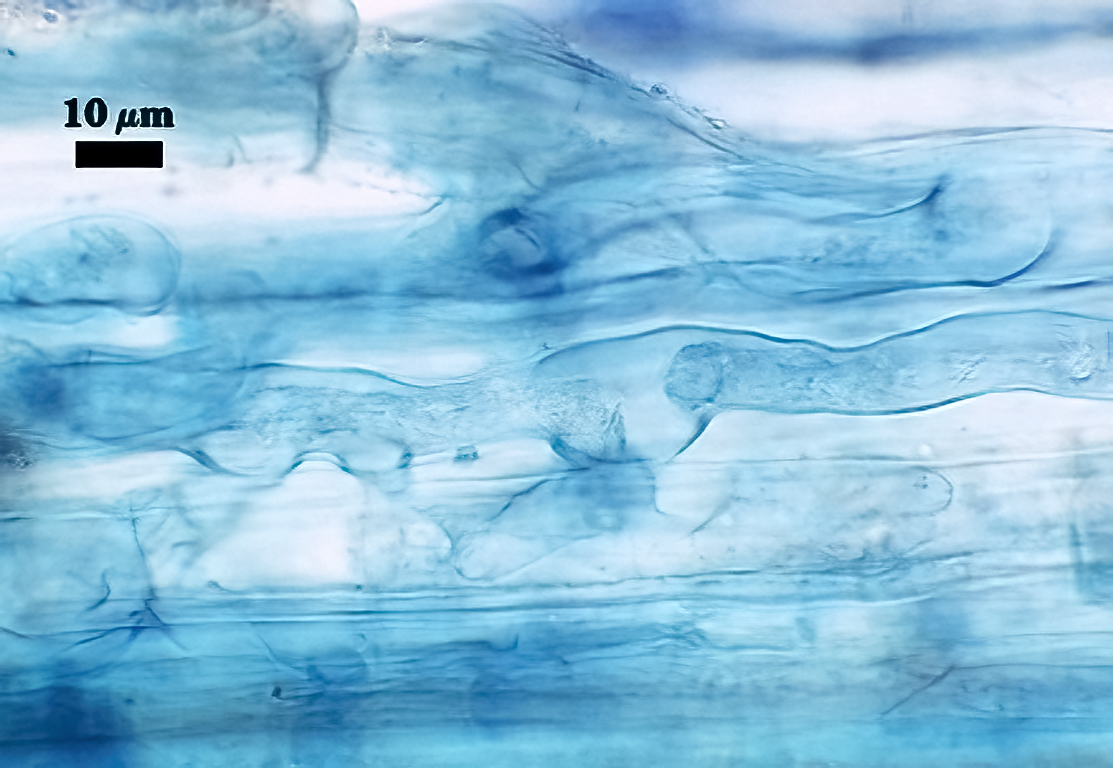 |
Notes
Sporocarps obtained directly from Maria Harrison were typical of the species, and contained not only mature thick-walled spores but also subhyaline to pale yellow spores with very thin spore walls. The latter spores were embedded in the hyphal matrix.
When sporocarps were used as inoculum to set up new cultures, only spores borne singly or in small aggregates were observed in 4-month-old sudangrass cultures. These spores bore little resemblance to those in mature sporocarps, being white to pale orange-brown, and with a much thinner spore wall. Clearly, spore phenotype is pleiomorphic, depending on the manner in which spores develop and their age.
Mycorrhizal morphology (faint staining, hyphal coiling, absence of vesicles) more closely resembles that of fungi in Paraglomeraceae than that of Glomeraceae, clearly separating this clade morphologically from other glomoid clades.
This species was synonymized with Glomus versiforme by Berch and Fortin (1983), but a re-examination of morphological characters supplemented with rRNA gene sequence data provided evidence that G. epigaea is a separate species (Schüßler et al., 2011). The reference culture of G. epigaea has provenance (albeit through may culture generations) with the original material propagated by Barbara Hetrick when she worked at Kansas State University.
The images below can be uploaded into your browser by clicking on the thumbnail or can be downloaded to your computer by clicking on the link below each image. Please do not use these images for other than personal use without expressed permission from INVAM.
High Resolution Images | |||
|---|---|---|---|
 |  |  |  |
 |  |  |
|
Links to Gene Sequences in Genbank
Reference
- Berch, S. M. and J. A. Fortin. 1983. Lectotypification of Glomus macrocarpum and proposal of new combinations: Glomus australe, Glomus versiforme, and Glomus tenebrosum (Endogonaceae). Can. J. Bot. 61:2608-2617.
- Daniels, B. A. and J. M.Trappe. 1979. Glomus epigaeus sp. nov., a useful fungus for vesicular-arbuscular mycorrhizal research. Can. J. Bot. 57:539-542.
- Daniels, B. A. and J. A. Menge. 1980. Secondary sporocarp formation by Glomus epigaeus, a vesicular-arbuscular mycorrhizal fungus, in long-term storage. Mycologia 72:1235-1238.
- Schüßler A, M. Krüger M and C. Walker. 2011. Revealing natural relationships among arbuscular mycorrhizal fungi: culture line BEG47 represents Diversispora epigaea, not Glomus versiforme. PLoS ONE 6:e23333. doi:10.1371/journal.pone.0023333.