Acaulospora capsicula
(reference accession NC177)
= Acaulospora colossica
Whole Spores
Below photo here shows appearance of spores from the time they first begin to form (2) on a sporiferous saccule (1) until they mature and become sessile (3-5).
| Spore Growth | |
|---|---|
 | 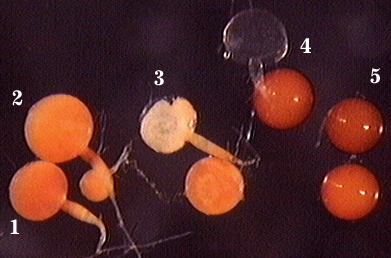 |
 COLOR: Orange-brown (20-80-80-0) to dark red brown (0-20-60-0). The median color is reddish-orange (0-50-60-0).
COLOR: Orange-brown (20-80-80-0) to dark red brown (0-20-60-0). The median color is reddish-orange (0-50-60-0).
SHAPE: Globose, subglobose.
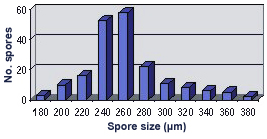
SIZE DISTRIBUTION: 180-380 µm, mean = 279 µm (n = 110)
Subcellular Structure of Spores
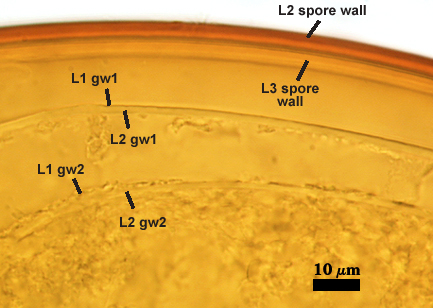
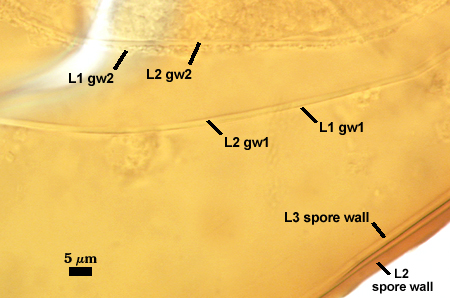
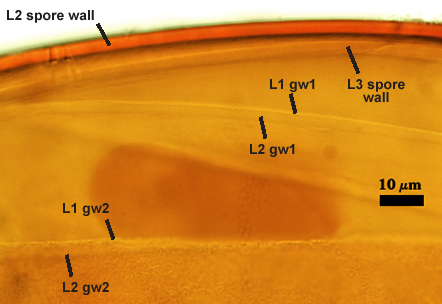
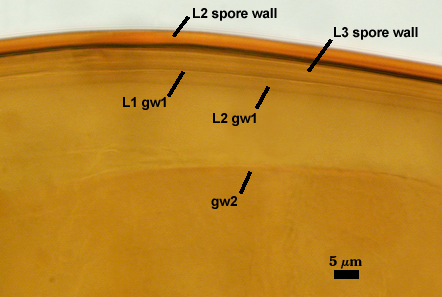
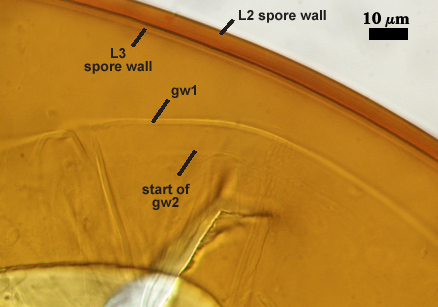
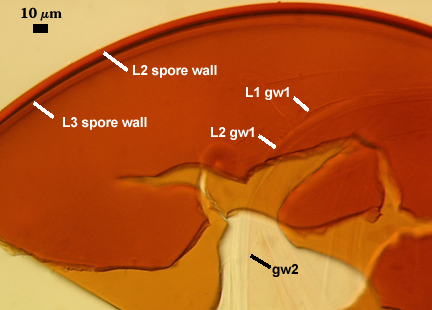
SPORE WALL: Three layers (L1, L2, and L3), the outer one continous with the wall of the neck of the parent sporiferous saccule and inner two synthesized as the spore expands from the saccule neck. L2 and L3 are formed sequentially during spore wall differentiation (see developmental sequence below, from left to right).
| Developmental Sequence | ||||
|---|---|---|---|---|
 | 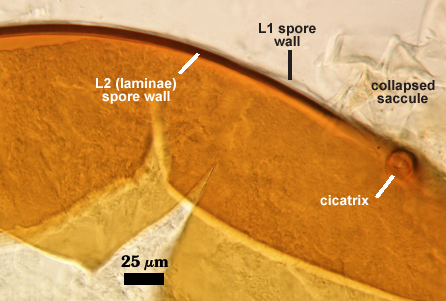 | 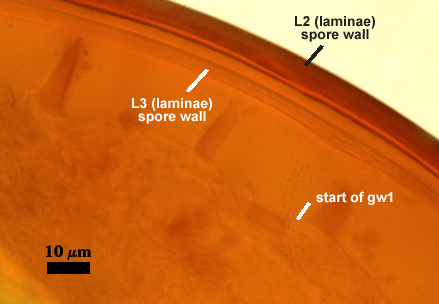 |  |  |
L1: Hyaline, smooth, 1.6-3.2 µm thick when intact on juvenile spores; continuous with the wall of the saccule neck. This layer degrades or sloughs as the spore dehisces from the saccule neck.
L2: A layer consisting of very fine and often adherent sublayers (= laminae), orange (0-60-100-10) to orange-brown (0-40-50-10), 2.0-4.8 µm thick (mean = 3.25 µm); appearing a darker orange-brown in Melzer’s reagent; often adherent to the outer layer (L1) when it is present.
L3: Another layer consisting of very fine and often adherent sublayers (= laminae), light brownish-yellow (0-10-40-0) usually, 1.6-4.8 µm thick (mean = 2.5 µm); no reaction in Melzer’s reagent. This layer may be hard to see with brightfield optics because it is close in color to L2 and when adherent, boundaries between layers are harder to see.
| Spores mounted in PVLG | |
|---|---|
|
|
| Spores in PVLG & Melzer’s reagent | |
|---|---|
|
|
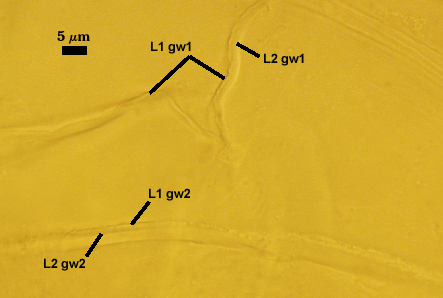
GERMINAL WALLS: Two hyaline flexible inner walls (gw1 and gw2) are present.
GW1: Two tightly adherent layers (L1 and L2) are formed. L1 is very thin, rarely exceeding 0.5 µm in thickness. L2 is 1.0-2.4 µm thick. Neither layer produces a reaction in Melzer’s reagent.
GW2: Two tightly adherent layers (L1 and L2) of near equal thickness are formed. L1 is 0.5-0.8 µm thick and coated on the surface with granular excresences (or “beads”) that tend to become dislodged and float away when pressure is applied to it. These “beads” are stabilized after preservation in formalin, but otherwise may be absent on mounted spores within a few weeks to months of storage. L2 is 0.5-1.0 µm thick and generally is nonreactive in Melzer’s reagent. In some spores, L2 will produce a very pale pink color (0-20-10-0) in Melzer’s reagent.
Cicatrix
Circular to ovoid scar with raised edges formed by L2 of the spore wall, 10-17 µm in diameter (mean = 14.6 µm). This scar shows the occlusion (growth of L2 and L3 of the spore wall) separating contents of the spore with that of the neck of the sporiferous saccule.
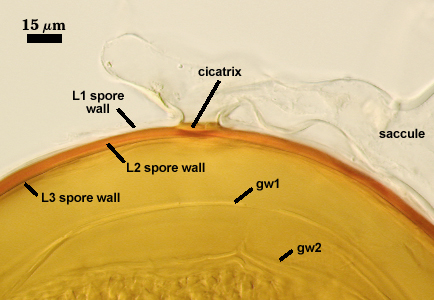
Pedicel
A short hyphal branch, or “pedicel”, links spore with the neck of the sporiferous saccule when they are attached. The intact pedicel is 2.5-6.5 µm long and consists of L1 of the spore wall and the wall of the saccule neck (which are continous with each other). Breakage of the pedicel usually results in detachment of spore from saccule neck.
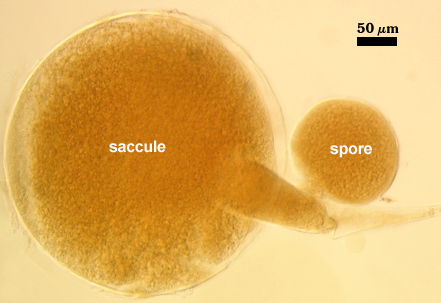
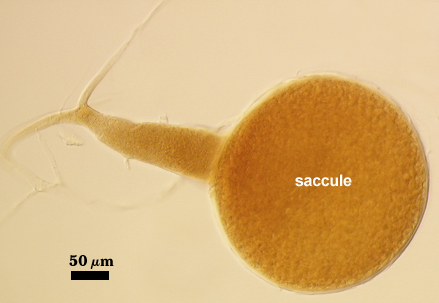
Sporiferous Saccule
COLOR: Hyaline when fully developed, light pink with opaque contents when young.
SHAPE: Mostly globose
SIZE: 240-360 µm, mean = 295 µm. Full size is attained prior to initiation of spore differentiation.
SACCULE WALL: One layer, smooth surface, 3.2-6.4 µm thick (mean = 3.9 µm).
DISTANCE FROM SACCULE TO SPORE: 88-160 µm (mean = 127 µm)
Mycorrhizae
Arbuscules and intraradical hyphae stain only faintly in acidic blue stains (trypan blue, direct blue), so that colonization is barely detectable under a stereomicroscope. Only with a compound microscope are details of colonization evident. Infection units rarely overlapping, often patchily distributed in sorghum roots. Intraradical hyphae usually coiled within cortical cells, 4-12.5 µm wide. No intraradical vesicle formation was evidenced in the pot culture host examined.
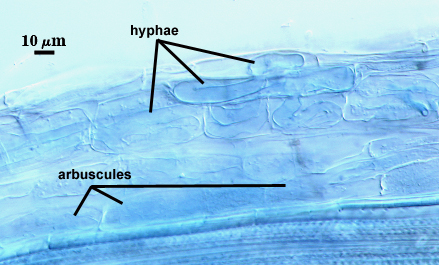
The staining reaction of mycorrhizae by Blaszkowski (1990) in his description of A. capsicula was “intense staining”. This variation, at present, cannot be explained (fungal genotype or staining method or physiological state of the different host species).
Germination
From hyaline, fragile (too much so to define boundaries in the spore examined) pregermination orb that forms on the surface of the innermost germinal wall (gw2).
Notes
This reference culture was the source of spores for description of A. colossica, which are among the largest formed within Acaulospora, and are comparable only to those of A. foveata and A. tuberculata. Otherwise, they are similar in appearance (smooth surface, similar color) to spores of A. laevis and A. koskei. Subcellular structure and organization in spores also is similar to that of A. laevis and A. koskei (Schultz et al., 1999). All of these species have three layers in the spore wall, with those of A. colossica and A. laevis almost identical. The innermost layer of the spore wall of A. koskei is distinct in producing a dextrinoid reaction in Melzer’s reagent.
Now with more accessible information on species from Poland, morphological data indicates likely synonymy between A. colossica and Acaulospora capsicula (Blaszkowski, 1990) with the latter name having priority.
Reference
- Blaszkowski, J. 1990. Polish Endogonaceae. VII. Acaulospora capsicula sp. nov. Mycologia 82:794-798.
- Schultz, P. A., J. D. Bever, and J. B. Morton. 1999. Acaulospora colossica sp. nov. from an old field in North Carolina and morphological comparisons with similar species, A. laevis and A. koskei. Mycologia 91:676-683.