Acaulospora scrobiculata
(reference accession BR984)
Whole Spores
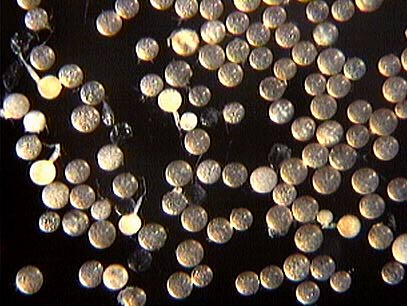
 COLOR: Many subyaline to pale yellow (0-0-10-0), but some darker spores are straw-colored (0-10-20-0).
COLOR: Many subyaline to pale yellow (0-0-10-0), but some darker spores are straw-colored (0-10-20-0).
SHAPE: Globose, subglobose, occasionally irregular.
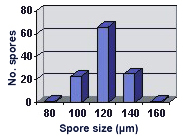
SIZE DISTRIBUTION: 80-160 µm, mean = 120.3 µm (n = 116).
Subcellular Structure of Spores
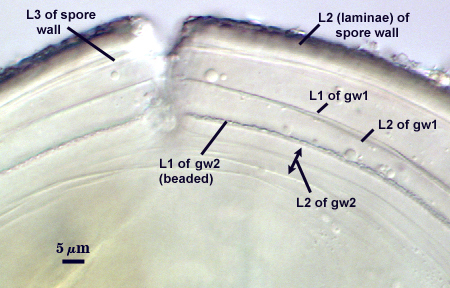
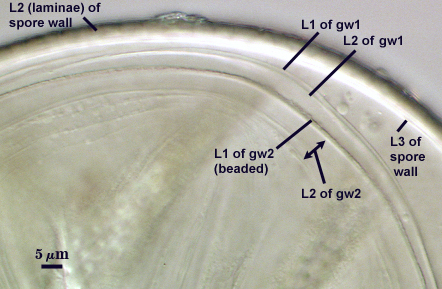
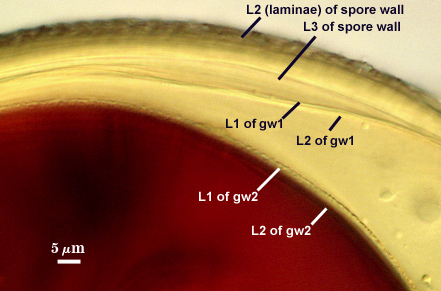
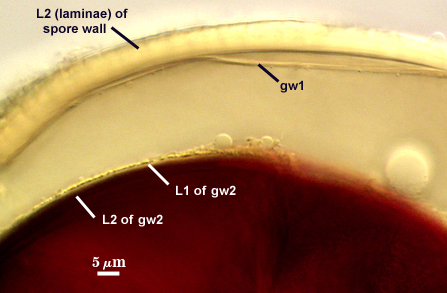
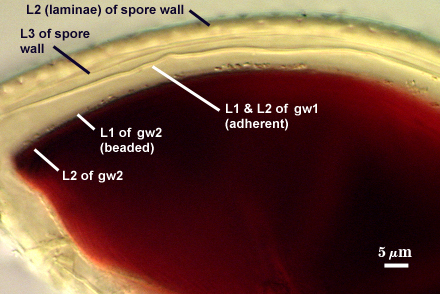
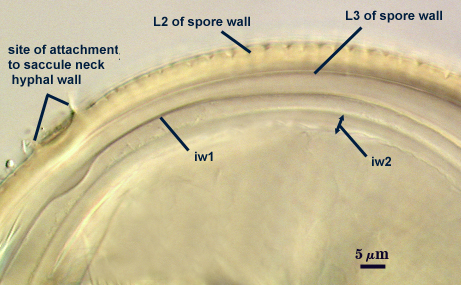
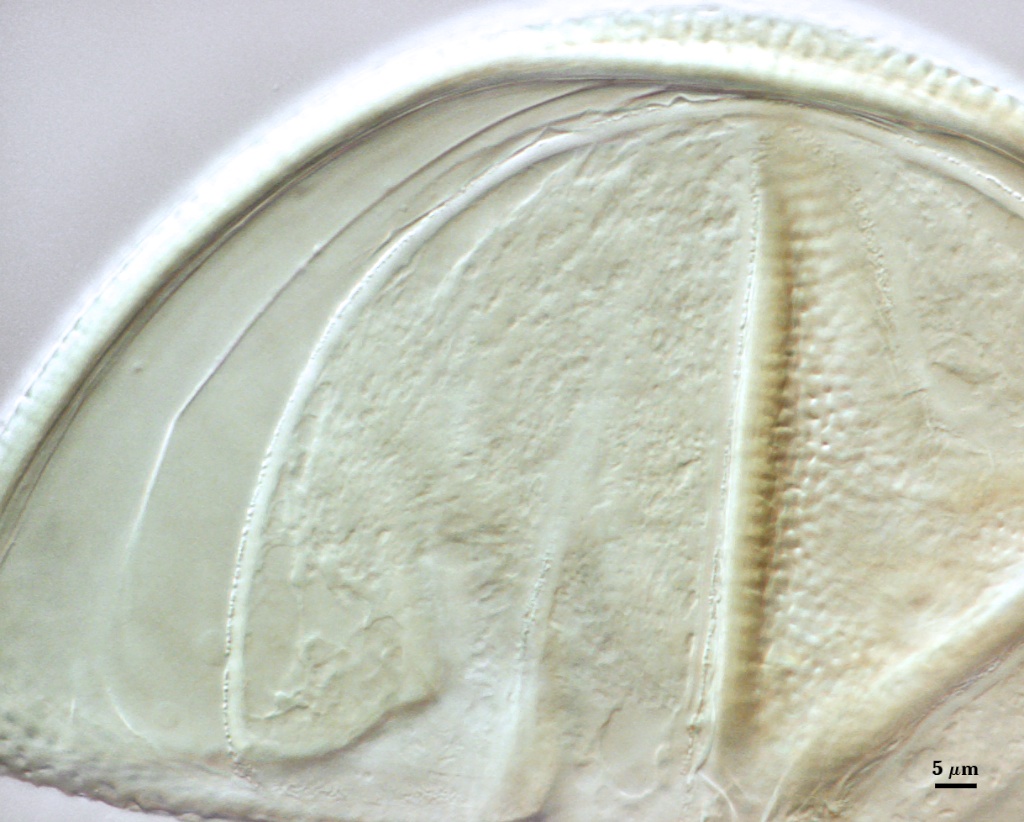
SPORE WALL: Three layers (L1, L2, and L3), the outer continous with the wall of the neck of the parent sporiferous saccule and the latter two being synthesized with origin of the spore.
L1: Hyaline; 0.8-1.2 µm thick; degrading and sloughing early in spore wall differentiation, often absent on mature spores.
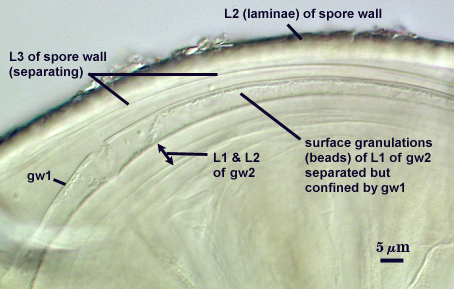
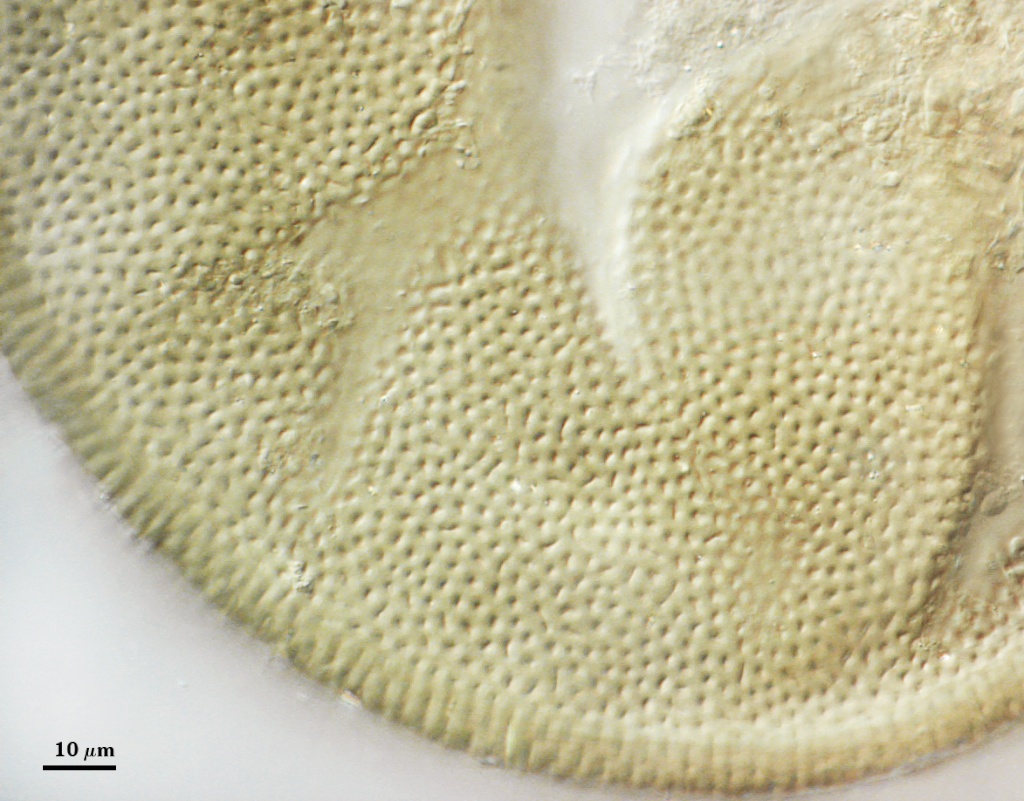
L2: A layer that thickens initially by formation of pale yellow (0-0-20-0) to tan (5-0-20-0) sublayers (or laminae) with ovoid concave depressions on the surface. These depressions are 0.6-2.0 µm across and 0.5-1.4 µm deep. Some merge together to form channels 5-12 µm long. Sublayers sometimes appear to split and consist of two groups of near-equal thickness, one including depressions and the other underlying the depressions, composite thickness 4.5-7 µm (mean of 6.1 µm).
L3: A layer designated as a discrete component of the spore wall only because it sometimes separates slightly from the spore wall and has defined boundaries. When separated, it can be interpreted as a separate flexible inner wall (which we have done until recently). However, it is considered to be part of the spore wall because it usually is integral (adherent) and sometimes shows evidence of just being another sublayer (lamina) of the spore wall. It is analagous to L3 found in other species with ornamented spore walls, such as A. rehmii and A. tuberculata, and species with a smooth spore wall, such as A. mellea. In some other species (e.g. A. koskei and A. laevis) this layer truly is distinct and has sublayers much like L2.
| Spores mounted in PVLG | ||
|---|---|---|
|
|
|
| Spores mounted in PVLG and Melzer’s reagent (1:1 v/v) | |||
|---|---|---|---|
|
|
|
|
GERMINAL WALLS: Two flexible hyaline inner walls (gw1 and gw2) can be seen in all spores IF they separate when each spore is broken.
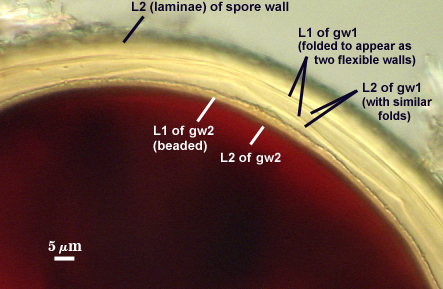
GW1: Two tightly adherent hyaline layers are formed (L1 and L2), both of near equal thickness, together measuring 1.0-1.3 µm. Neither react in Melzer’s reagent. This wall often is positioned close to the spore wall except in vigorously crushed spores and occasionally may not even be easy to detect. In other spores, iw1 may produce one to many folds, thus giving the appearance of a bewildering array of flexible inner walls and making diagnosis difficult.
GW2: Two tightly adherent hyaline layers formed (L1 and L2). L1 is 0.6-1.2 µm thick, with granular excresences (or “beads”) that tend to become dislodged and float away with applied pressure. These “beads” are stabilized after preservation in formalin, but otherwise may be absent on mounted spores within a few months of storage. L2 is 1.2-4.1 µm thick and stains light purplish pink (0-60-30-0) to reddish-brown (20-80-60-0) in Melzer’s reagent (brownish appearance usually is due to viewing gw2 through the yellow pigments of the spore wall).
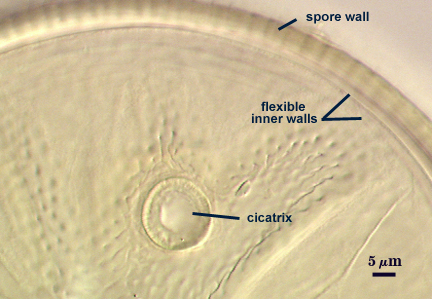
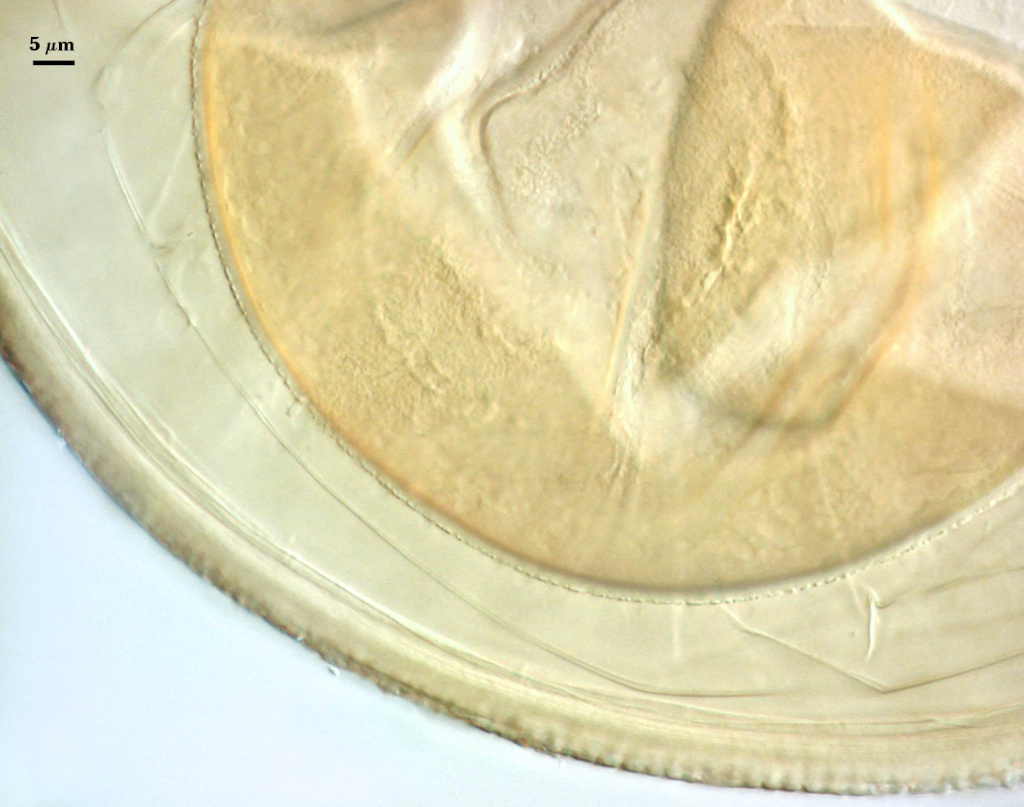
Cicatrix
A circular to ovoid scar indicating region of contact between spore and saccule neck during spore synthesis; the depression within the scar is smooth, 7.5-13.4 µm in diameter (mean = 10.7 µm). The connection between saccule neck and spore is somewhat complex in that it initially consists of only the L1 layer of the spore (continuous with the wall of the saccule neck), followed by synthesis of sublayers of the L2 (laminate) layer which form between saccule neck and spore. After degradation and sloughing of L1, these sublayers form a ridge 2.8-4 µm high around the occluded pore (right photo below). Mode of formation of this region is analagous to that which occurs during synthesis of spore wall layers in Glomus species.
| Scrobiculata depression | |
|---|---|
|
|
Sporiferous Saccule
COLOR: Hyaline
SHAPE: Globose, subglobose.
SIZE: 100-230 µm, mean = 114.3 µm.
SACCULE WALL: One hyaline layer, smooth surface, 1.4-2.0 µm thick.
DISTANCE 40-80 µm.
Germination
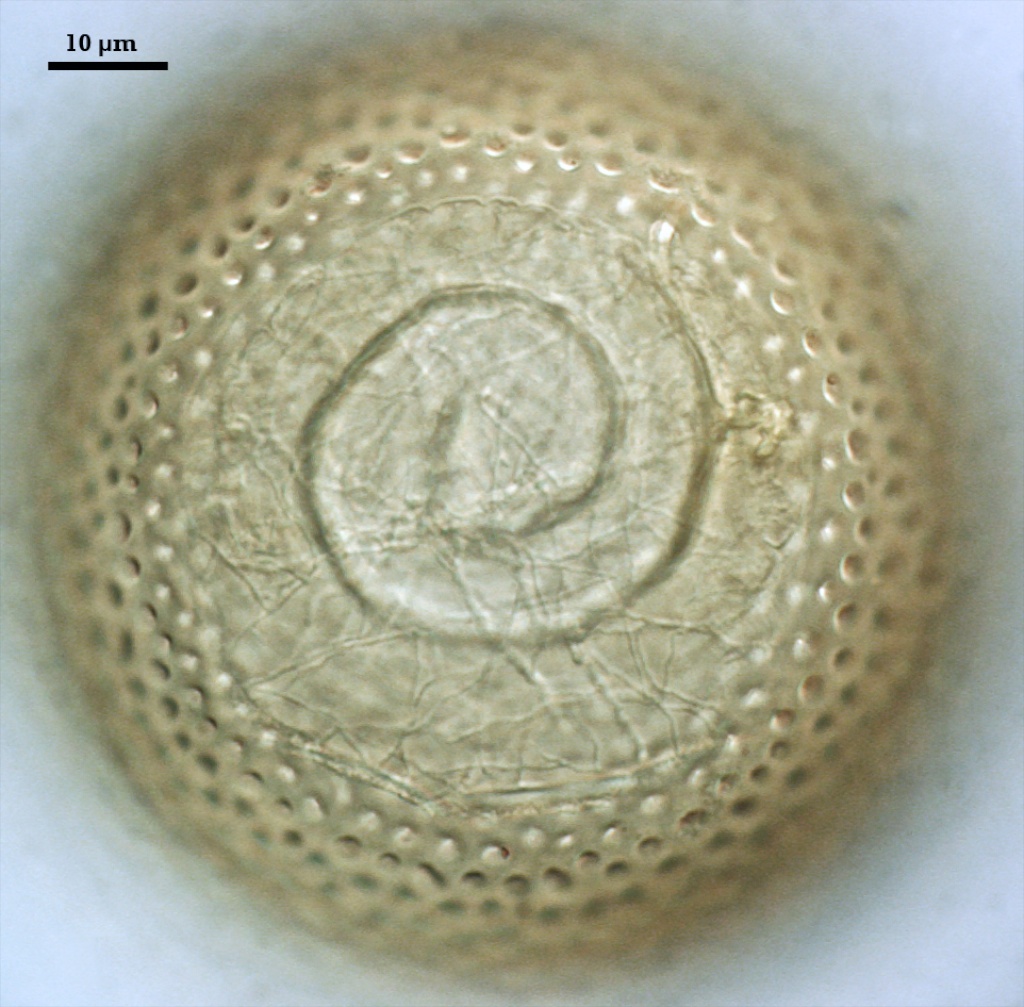
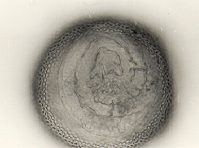 An ovoid “germination orb” forms on the innermost germinal wall (gw2), from which germ tubes form and penetrate through the spore wall. This orb is difficult to see except in older spores where contents have cleared with fusion of lipid globules in the spore lumen, mostly because it is wide enough to span most of the diameter of the spore and so edges of the orb are seen only with a limited range of spore orientations. Spain (1992) reports orbs of A. scrobiculata are slightly ovoid with an inward spiral configuration. The photo above was provided courtesy of Joyce Spain from her publication.
An ovoid “germination orb” forms on the innermost germinal wall (gw2), from which germ tubes form and penetrate through the spore wall. This orb is difficult to see except in older spores where contents have cleared with fusion of lipid globules in the spore lumen, mostly because it is wide enough to span most of the diameter of the spore and so edges of the orb are seen only with a limited range of spore orientations. Spain (1992) reports orbs of A. scrobiculata are slightly ovoid with an inward spiral configuration. The photo above was provided courtesy of Joyce Spain from her publication.
Mycorrhizae
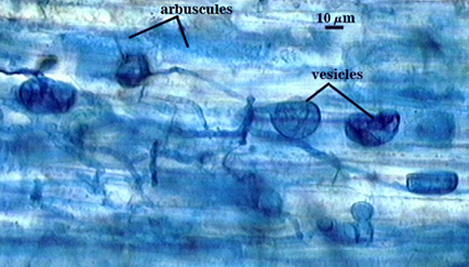
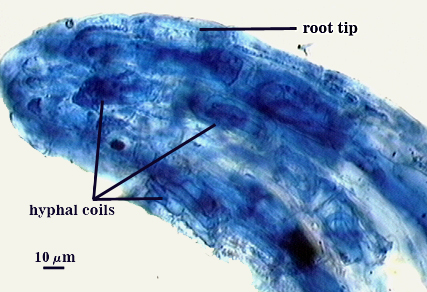
Arbuscules often stain lightly in trypan blue, but sometimes stain more darkly; intensity of staining appears to vary with many factors that have yet to be resolved (possibly age of the mycorrhizae and of the host root). Intraradical hyphae tend to be thicker (4-6 µm) and form more coils near point of entry into a root. Infection units are patchy because they often do not overlap and appear merged. “Foraging” hyphae grow along the root axis, parallel to each other, and generally 2-4 µm in diameter. Vesicles often form most abundantly near entry points and range from spherical to oblong to irregularly shaped. Note the last photo in the sequence below, in which the tip of a corn root is filled with many coiled hyphae. The fungus and host respond dynamically to structural and physiological changes in root regions, and this accounts for the wide range of morphological structure and pattern in the same root system.
| Arbuscules in cortical cells of corn roots | ||
|---|---|---|
|
|
|
| All mycorrhizal structures in corn | |
|---|---|
|
|
Notes
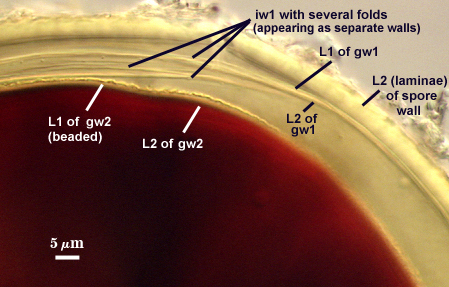
Inner wall structure often is very difficult to define clearly for several reasons: (1) ornamentations on L2 of the spore wall cause considerable light-scattering which reduces the ability to resolve hyaline flexible walls within the spore and (2) L3 of the spore wall and iw1 can produce numerous folds that make it almost impossible to define inner wall structure clearly. We have only gotten the “hang of it” in the past year from examining hundreds of slides. The trick is to focus first on the second germinal wall (gw2) as a positional reference; its easy to start with because the wall consistently separates readily from the rest of the spore and has a beaded surface (if examined at least within 30 days of mounting). Then proceed back to the spore wall. If there are many folds, ignore that spore and move to another one. When only a few folds are present, usually they can be traced back to an area where they merge and no folds are seen (see photos in Melzer’s reagent above).
Schenck and co-workers appeared to have considerable difficulty with diagnosis of Acaulospora species in part because of confusion concerning the “beaded” ornamentation on gw2 of A. spinosa and many other species. When it was not detected, the spores were classified as being a separate (usually undefined) species. It should be remembered that the granules on L1 of gw2 are not permanently fixed in place (unless spores have been stored for more than 60 days in formalin or some other preservative) and can disperse upon crushing of the spore and disappear. They also appear to dissolve or lose refractivity in PVLG-based mountants after 30-90 days (varying with condition of spores and fungal species).
The images below can be uploaded into your browser by clicking on the thumbnail or can be downloaded to your computer by clicking on the link below each image. Please do not use these images for other than personal use without expressed permission from INVAM.
High Resolution Images | |
|---|---|
 |  |
 |  |
 |  |
Reference
- Spain, J. L. 1992. Patency of shields in water-mounted spores of four species in Acaulosporaceae (Glomales). Mycotaxon 43:331-339.